DATE2022.02.08 #Press Releases
Birth: Diamond's twin brother
The smallest cage unit in the new chiral carbon network
Disclaimer: machine translated by DeepL which may contain errors.
Hiroyuki Isobe, Professor, Department of Chemistry
Kohki Ikemoto, Lecturer, Department of Chemistry
Key points of the presentation
- The "twin brother of diamond (PORX)" appeared in a chemical synthesis. Pollux is a new material that has the same "perfect symmetry" and "strong isotropy" as diamond, and also has a unique "chirality," but until now it has been a theoretical or imaginary material.
- In this research, we designed and synthesized the "smallest cage unit of poluxenes" called poluxenes.
- The chemical synthesis has revealed the unique "chirality" characteristics of poluxenes.
Announcement Summary
A research group led by Professor Hiroyuki Isobe of the Graduate School of Science, The University of Tokyo, has made the world's first appearance of "Pollux," a twin brother of diamond, through chemical synthesis (Figure 1). They designed and synthesized the molecule "Polluxen," the smallest cage unit cut out from Pollux, which has the same "perfect symmetry (Note 1)" and "strong isotropy (Note 2) " as diamond (Figures 2 and 3). It was theoretically known that polux has a unique chirality (Note 3), which is different from that of diamond, but this research enabled the structural analysis of poluxene as a real substance for the first time and clarified part of its complex chirality characteristics. These results are expected to lay the foundation for the development of new chiral nanocarbon materials in the future.
The research results were published in the international journal "Proceedings of the National Academy of Science, U.S.A. (PNAS )" on February 8, 2022.
Publication details
The beauty of diamonds attracts many people, but the beauty of their structure also fascinates scientists. For example, the smallest cage unit of diamond is known as adamantane, and its theoretical prediction in 1905/1924, isolation from crude oil in 1933, the first chemical synthesis in 1941, and the establishment of a simple mass synthesis method in 1957 have greatly advanced research in the fields of physics and chemistry. The beauty of the diamond structure has been mathematically elucidated very recently, and it has been proposed that the three-dimensional space is filled with carbon atoms in such a way that it has "perfect symmetry" and "strong isotropy". Based on the mathematical approach that clarified "perfect symmetry" and "strong isotropy," it was proposed in 2008 that there can be only one more carbonaceous material with "perfect symmetry" and "strong isotropy" other than diamond. In fact, the unique network structure of this dream new material, the "twin brother of diamond," has a long history and has been the subject of frequent theoretical studies since its first proposal in 1932, and has been given various names (Laves' net, Net 1, Y*, net (10,3)-a 3/10/c1, srs, double triamond, K4). One of the reasons this network has attracted widespread interest is the presence of a unique "chirality" not found in diamonds.
However, until now, the "diamond's twin brother composed of carbon" has been a theoretical and imaginary material, and its unique network structure has never existed as a real material that can be handled (Figure 1).

Figure 1: Pollux and Polluxen (smallest cage unit in orange). Polluxen, shown in orange, was chemically synthesized.
The research team has successfully chemically synthesized the smallest cage unit of these "diamond twin brothers" (Figures 2 and 3). The research team also named the infinite-length carbon network Pollux and the smallest cage unit Polluxene, after the Gemini twin brothers of Castor and Pollux in Greek mythology.
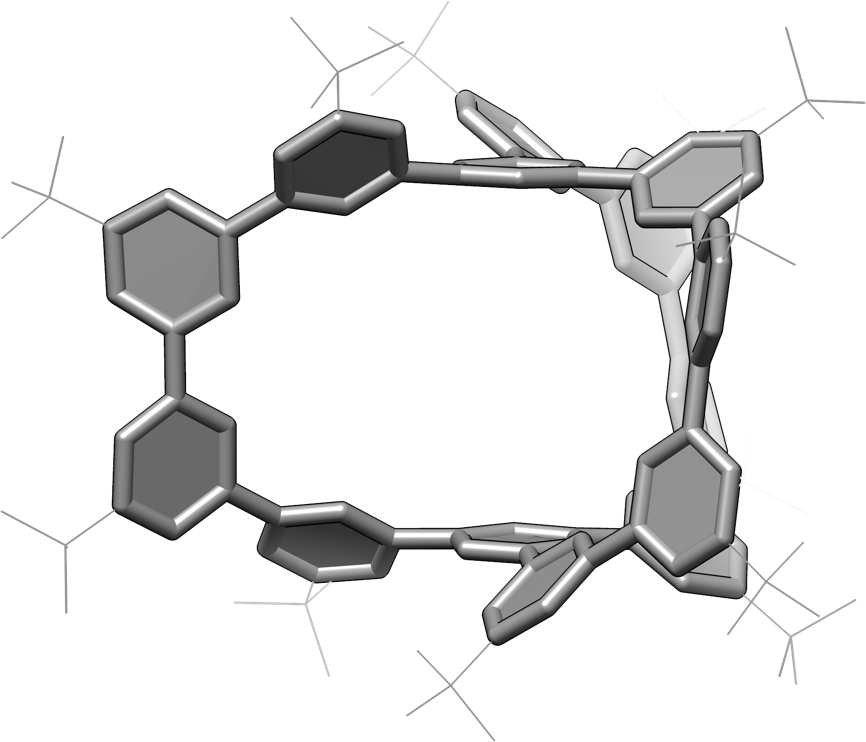
Figure 2: Polluxene. Side view of the crystal structure of chemically synthesized poluxene.
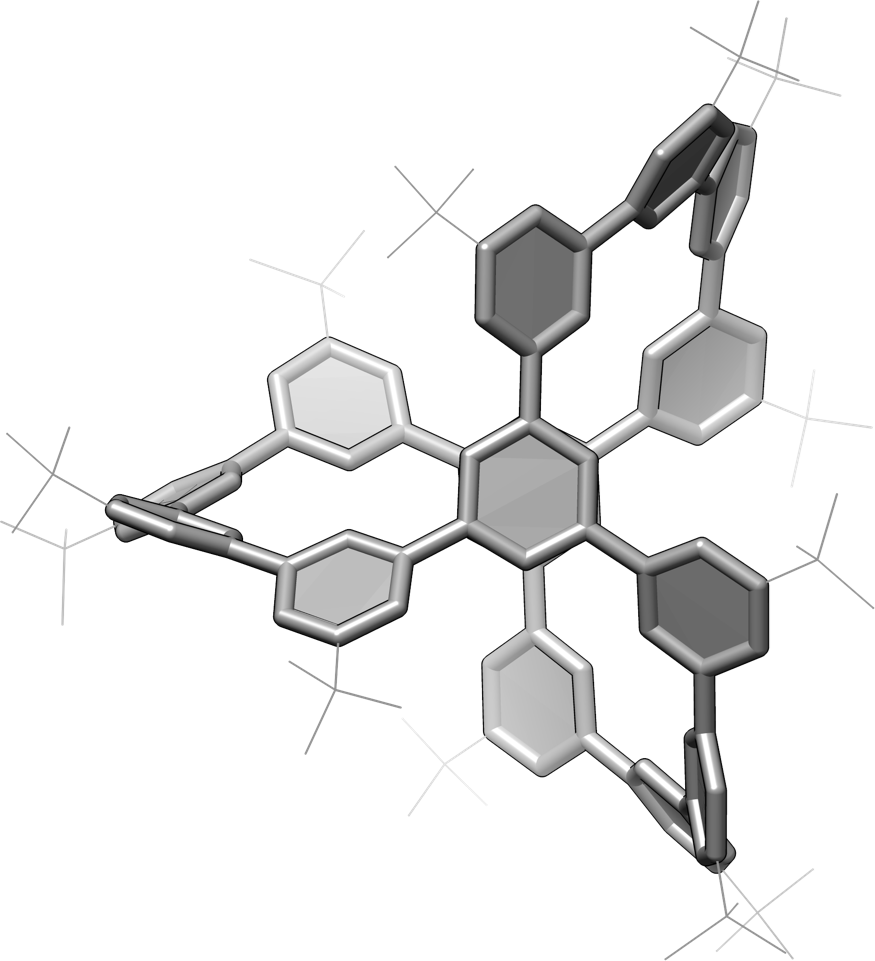
Figure 3: Polluxene. Crystal structure of chemically synthesized poluxene, viewed from above.
The polxene is the smallest cage unit that maintains the symmetry of the porcus, and in this study, a new design was conceived that places benzene rings at the 14 vertices of the cage structure. The original porcus was proposed as a network of sp2-mixed carbons in a planar triangular basic structure, and the idea was to replace it with a benzene ring. Based on this idea, the research group has succeeded in the chemical synthesis of polxene by linking 14 benzene rings at the vertex with 15 carbon-carbon bond edges (Figures 2 and 3). In this synthesis, three types of reactions called aromatic coupling reactions were utilized, all of which are "Japan-originated" coupling reactions, and a simple and efficient synthetic method was realized.
In this research, we have further succeeded in unlocking the secret of the "chirality" of Pollux-Poluxen. It had been proposed that there existed a counter-palpability or chirality in the Pollux network, which is a mirrored relationship like "right hand/left hand". In this study, the research group synthesized Polksen's "without chirality" and "with chirality" separately, and further conducted theoretical and mathematical analysis. As a result, it was found that there are very complicated stereoisomers (Note 4) in the structure of Pollux-Poluxen, and the possible structures of the smallest cage unit are as many as 5600 species. Then, a method to fix chirality was devised, and two chiral species in a mirror-image relationship were chemically synthesized, and each was successfully isolated as a chiral substance.
The chemical synthesis of the imaginary substances "Pollux" and "Polluxen" is expected to contribute to the development of nanocarbon materials in the future. In particular, the existence of complex stereoisomerism and chirality has been demonstrated, which is expected to attract more attention as a chiral material.
This research was conducted as part of a Grant-in-Aid for Scientific Research.
| Name of Researcher | Affiliation |
| Toshiya Fukunaga | Graduate Student, Graduate School of Science, The University of Tokyo |
| Takahide Kato | Graduate Student, Graduate School of Science, The University of Tokyo |
| Kohki Ikemoto | Lecturer, Graduate School of Science, The University of Tokyo |
| Hiroyuki Isobe | Professor, Graduate School of Science, The University of Tokyo |
Journal
-
Journal name Proceedings of the National Academy of Sciences of the United States of America (PNAS) Title of paper A minimal cage of a diamond twin with chirality
(A minimal cage of a diamond twin with chirality)Author(s) Toshiya M. Fukunaga, Takahide Kato, Koki Ikemoto* & Hiroyuki Isobe* (authors) DOI Number 10.1073/pnas.2120160119 URL http://dx.doi.org/10.1073/pnas.2120160119
Explanation of Terms
Note 1 Perfect symmetry
A structure is said to have symmetry if it remains unchanged after applying symmetry operations such as inversion or 180° rotation, and is said to have perfect symmetry if it has the maximum number of elements of symmetry in the crystal. A structure with perfect symmetry means that no matter how the components are rearranged, the symmetry does not increase any further. Diamond's carbon network has this perfect symmetry. ↑up
Note 2: Strongly isotropic
A property in which the distribution of the components of a crystal is independent of direction and appears the same when viewed from any angle. The carbon network of diamond has this strong isotropy. ↑up
Note 3 Chirality
Contrapositional nature. The property that mirrored images (mirror images) do not overlap each other, as in the case of the right and left hands. The presence of chirality is called chiral ("chiral" is derived from the Greek word for "hand"). ↑up
Note 4 Stereoisomerism
A relationship that has the same bonding mode but cannot be superimposed no matter how it is moved or rotated in space. ↑up
Reference
Please also refer to the following press release for a representative related previous study by Professor Hiroyuki Isobe et al.
Molecular Machines Packed with the Smallest Diamond Molecules in Tubular Molecules (August 25, 2021 )
World's First Nitrogen-Doped Nanotube Molecules (April 14, 2020 )
New Nanotubes (January 11, 2019 )
The University of Tokyo's Graduate School of Science presents a video introduction to its research (November 21, 2018 ).
... Axial Rotation by Relaying Hydrogen Bonds (September 17, 2018 )
... (Almost) Frictionless: Bearings in the Molecular World (May 15, 2018)
∙ The Right and Left Hands of Chiral Tubular Molecules (November 28, 2017 )
∙ Spontaneous and Self-Selective Assembly of Two-Wheeled Molecular Bearings (November 17, 2016 )
∙ Chemistry and Mathematics of Tubular Molecules (June 28, 2016)
-Naphthalene to Negative Electrode Materials for All-Solid-State Lithium-Ion Batteries (May 16, 2016)
∙ New Materials for Single-Layer OLEDs from Toluene (November 5, 2015 )
∙ Aging and Rotational Motion of Nanosized Comas (March 2, 2015 )
The secrets inside finite-length carbon nanotube molecules (May 27, 2014 ) ∙ Finite-length carbon nanotubes.
The finite length index of carbon nanotubes (January 22, 2014 )
Elongated Finite-Length Carbon Nanotube Molecules from Pigments (May 22, 2013 )
The world's first zigzag-type carbon nanotubes ( January 9, 2013 ).
The world's first selective chemical synthesis of helical finite-length carbon nanotube molecules (October 12, 2011 )


