Disclaimer: machine translated by DeepL which may contain errors.
Big Bang Elemental Synthesis through Laboratory Observations
Seiya Hayakawa, Project Assistant Professor, Center for Nuclear Study |
Eishin Yamaguchi (Lecturer, Center for Nuclear Study) |


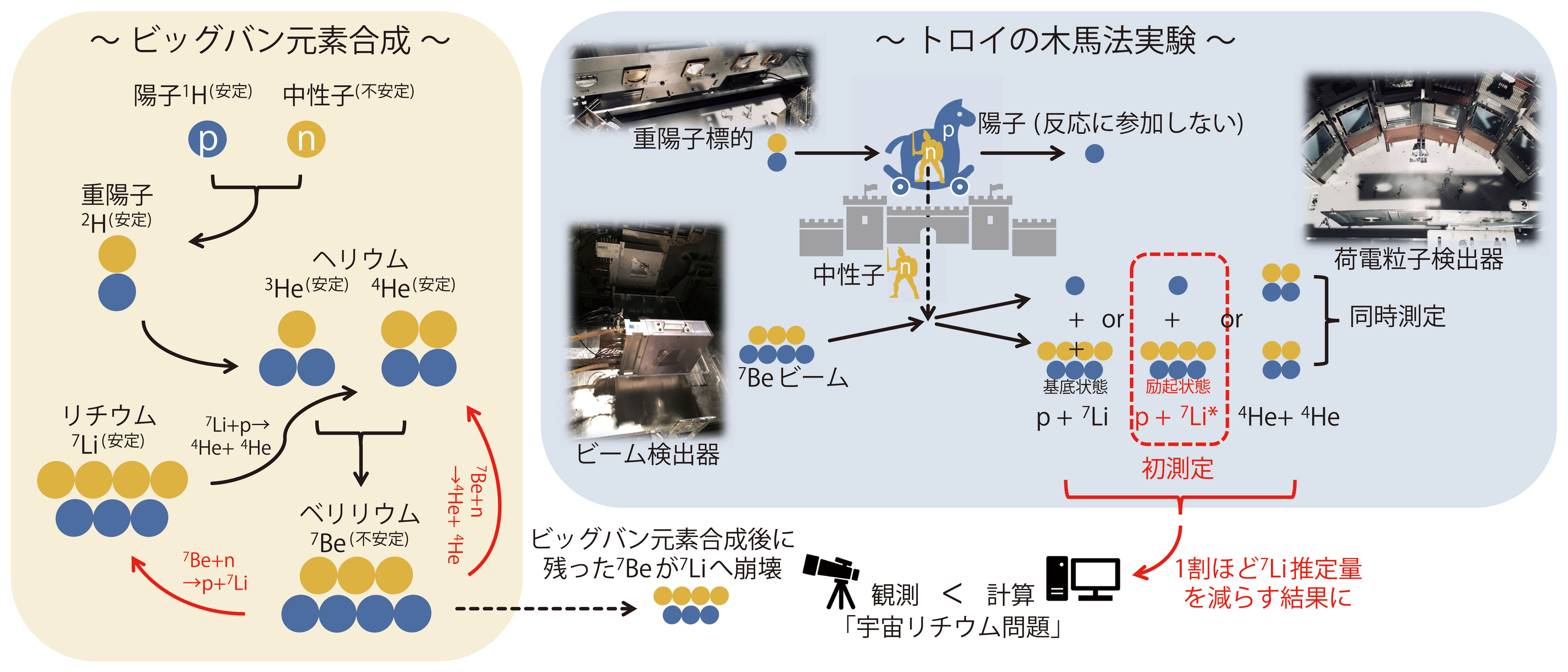
You may have a smartphone in your hand. Some of the lithium used in the battery was already synthesized within minutes after the start of the Big Bang 13.8 billion years ago. The Big Bang elemental synthesis, which began with the chaos of protons ( p) andneutrons (n), produced hydrogen and helium as its main products. Calculations of the production of their isotopes ( 1, 2H, 3, 4He ) agree remarkably well with observations, indicating the success of the Big Bang theory. On the other hand, the "cosmic lithium problem" has been an unsolved problem for many years, because the theoretical estimate of the amount of 7Li, which is a by-product of helium, is about three times larger than that of observation.
Is there a problem in estimating the amount of 7Li from observations of old stars? Are there physical phenomena that are not fully described by the standard Big Bang theory? To begin with, some reactions lack the thermonuclear reaction rate data necessary for elemental synthesis calculations, and experimental verification is still being conducted by nuclear physicists.
In the big bang elemental synthesis, 7Li readily reacts with a large number of protons present at the time of its production, and breaks up into two 4He atoms. On the other hand, 7Be produced from 3He+4He is an "unstable nucleus" that converts to 7Li with a half-life of 53 days, but can survive until after the big bang synthesis, which lasts only about 20 minutes. While the reactions that increase the amount of 7Be are relatively well understood, the 7Be+n reactions that decrease the amount of 7Be have attracted much attention in recent years. The main products are p+7Li or 4He+4He. The former seems to increase the amount of 7Li, but as mentioned above, 7Li is still "reduced" in the subsequent reaction with protons.
However, how can we measure the reaction between 7Be and neutrons, which are unstable nuclei? We applied an indirect method called the "Trojan horse method," in which a deuteron, which is a neutron and a proton combined, is used as a target and 7Be is injected as a beam. The name of this method is derived from the Greek myth of the Trojan horse, in which a soldier (neutron) is sent into the castle walls (target reaction) by hiding inside a wooden horse (deuteron). This experiment revealed for the first time that the contribution of the first excited state of 7Li in the 7Be+n→p+7Li reaction is about 10-15% of that of the ground state. This means that 7Be breaks down more than expected in the Big Bang. The application of the new thermonuclear reaction rate to the Big Bang elemental synthesis calculations has resulted in a downward revision of the estimated production of 7Li by about 10%.
Although we have not completely solved the cosmic lithium problem in this study, we have at least picked up information that we had missed until now in a relatively small-scale nuclear experiment. Now that one uncertainty has been resolved, further cooperation among astronomy, astrophysics, and nuclear physics is needed to solve the problem.
This work was published in S. Hayakawa et al, The Astrophysical Journal Letters 915, L13 (2021).
Published in the November 2021 issue of Faculty of Science News
Communicating to Faculty Research Students >