Disclaimer: machine translated by DeepL which may contain errors.
Lipids enter from the side and activate receptors
Wataru Shihoya, Assistant Professor, Department of Biological Sciences
Osamu Nureki, Professor, Department of Biological Sciences
Lipids are major components of cell membranes and form the boundary between self and non-self.
On the other hand, certain lipids function as signaling molecules by activating receptors on the membrane.
These lipid molecules are called lipid mediators, and failure of the system leads to various diseases.
We have determined the three-dimensional structure of a receptor bound to lysophosphatylserine, a type of lipid mediator,
transmitting a signal to the cell.
As a result, the mechanism by which the lipid enters from the lateral side of the receptor and activates the receptor was clarified.
![]()
Lysophosphatidyl serine (LysoPS) *note1 ) is a type of lipid mediator responsible for intercellular signal transduction and is produced in vivo by cleavage of one acyl group of phosphatidyl serine. LysoPS is being investigated as a potential countermeasure against cancer and infectious diseases because it modulates the function of the immune system by activating a receptor called GPR34. However, whether LysoPS is a true ligand for GPR34 is controversial, and it has not been clarified how LysoPS activates GPR34 at the molecular level.
Therefore, we studied the three-dimensional structure of LysoPS to elucidate why LysoPS can activate GPR34. We produced a large amount of the receptor protein and purified it to a high purity with LysoPS bound. We then mixed the complex with trimeric G proteinNote 2 ) to obtain a signaling complex in the activated state of the G protein (Figure, left). The complex was trapped in thin ice on a grid and photographed by cryo-electron microscopy. The structure of the complex was successfully determined by a technique called single particle analysis, in which 100,000 particles were extracted from the images taken.
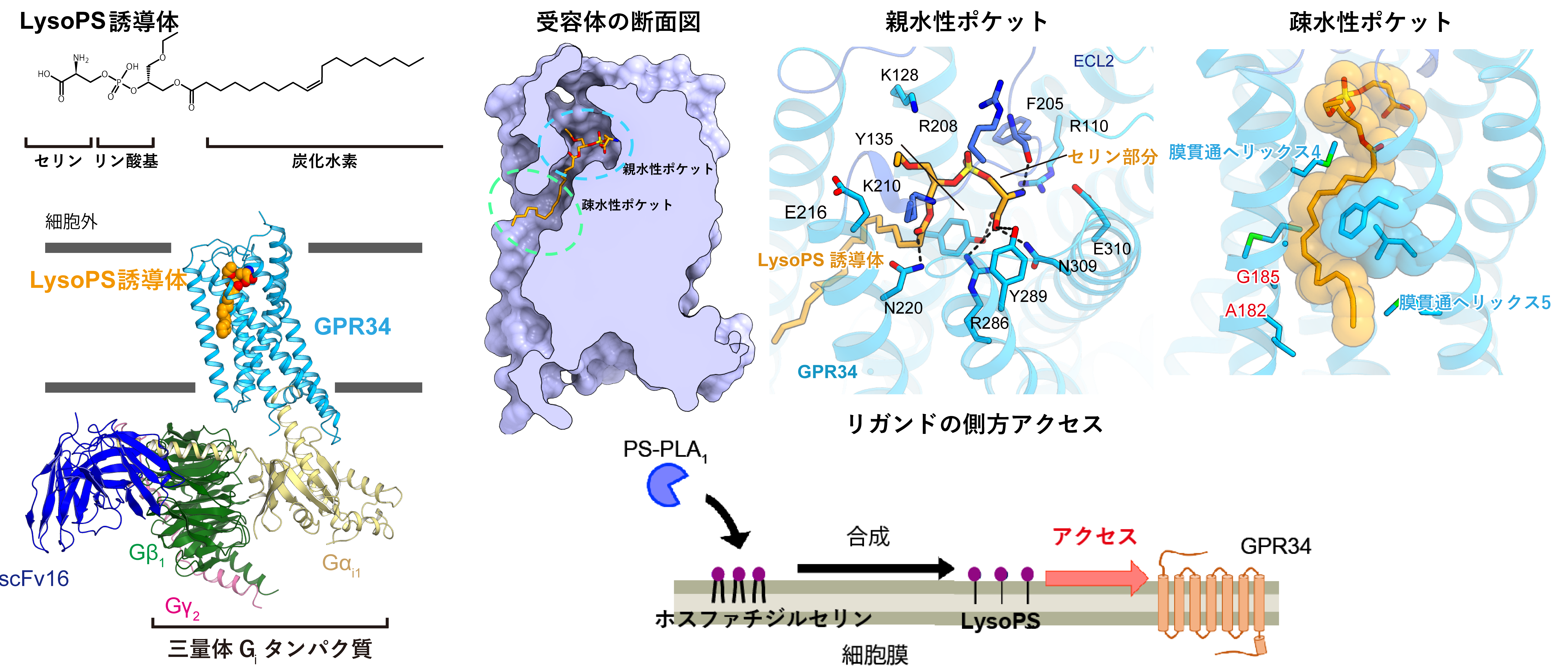 Overall structure of the GPR34 signaling complex. Left: Molecular structure of LysoPS and overall structure of the signaling complex. Top right: Cross section of the receptor pocket and interaction in hydrophilic and hydrophobic pockets. Bottom right: Model of receptor activation by PS-PLA1. The created lipid moves across the membrane and enters the receptor from the side.
Overall structure of the GPR34 signaling complex. Left: Molecular structure of LysoPS and overall structure of the signaling complex. Top right: Cross section of the receptor pocket and interaction in hydrophilic and hydrophobic pockets. Bottom right: Model of receptor activation by PS-PLA1. The created lipid moves across the membrane and enters the receptor from the side.
The ligand-binding pocket of GPR34 was composed of a hydrophilic pocket that recognizes the ligand head and a hydrophobic pocket that accommodates the hydrocarbon group (Figure, upper right). In the hydrophilic pocket, the serine moiety of LysoPS was tightly recognized by forming hydrogen bonds with polar amino acid residues. This serine-specific interaction may be the reason why GPR34 accepts and signals only LysoPS among various lipids. The ligand-binding pocket of GPR34 opened laterally through this groove, suggesting that LysoPS may move across the plasma membrane via intercellular adhesion, and that the hydrocarbon groups are accommodated in the hydrophobic pocket in the groove formed by the fourth and fifth transmembrane helicesNote 3). In fact, GPR34 is activated by LysoPS-producing enzyme (PS-PLA1) without the addition of LysoPS from the outside. This result indicates that the access of LysoPS from the plasma membrane side, not from the extracellular pathway, is important for the function of GPR34 (Figure, lower right), and is a major breakthrough in the receptor research.
The results of this study were published in T. Izume, et al. Nat. Commun. 81, 3205 (2024).
Note 1) LysoPS: In this study, a derivative of LysoPS was used for structural analysis.
Note 2) Trimeric G protein: A heterotrimeric protein that plays an important role in cell signaling. Upon binding to the receptor, the trimer is dissociated and interacts with downstream proteins to transmit signals.
Note 3) Transmembrane helix: A helical structure called an α-helix is formed when a portion of a protein passes through the cell membrane.
(Press release, February 7, 2024)
Published in The Rigakubu News May 2024
Frontiers of Science for Undergraduates >


