DATE2024.02.07 #Press Releases
Structure of the active form of the LysoPS receptor involved in immune responses
--contributing to drug discovery research by elucidating the binding mode of agonists--
February 7, 2024
A research group at the University of Tokyo, led by Professor Osamu Nureki from School of Science, in collaboration with Professor Tomohiko Ohwada and Junken Aoki of Graduate School of Pharmaceutical Sciences, activated lysophosphatidylserine (LysoPS) by a non-natural derivative and a metabolically stable agonist with the lipid moiety replaced by an aromatic one, The steric structure of the signalling complex between GPR34 and trimeric G protein Gi was determined by single-particle analysis using cryo-electron microscopy.
LysoPS is responsible for intercellular signalling, and GPR34, which has been reported to be one of the LysoPS receptors, is expressed on a wide range of cells, including immune cells, and is involved in functions such as tissue repair and suppression of infection. However, it was unclear what LysoPS molecular species activate GPR34 and how LysoPS is produced, passed on to GPR34 and activated.
The two structures determined revealed ligand-binding pockets open in the plasma membrane and the characteristic binding mode of agonists, including natural forms of LysoPS.
It was also shown that LysoPS produced on the plasma membrane directly accesses the pocket inside the receptor from the side of GPR34. The results of this study will enable the design of more effective agonists and contribute to drug discovery research.
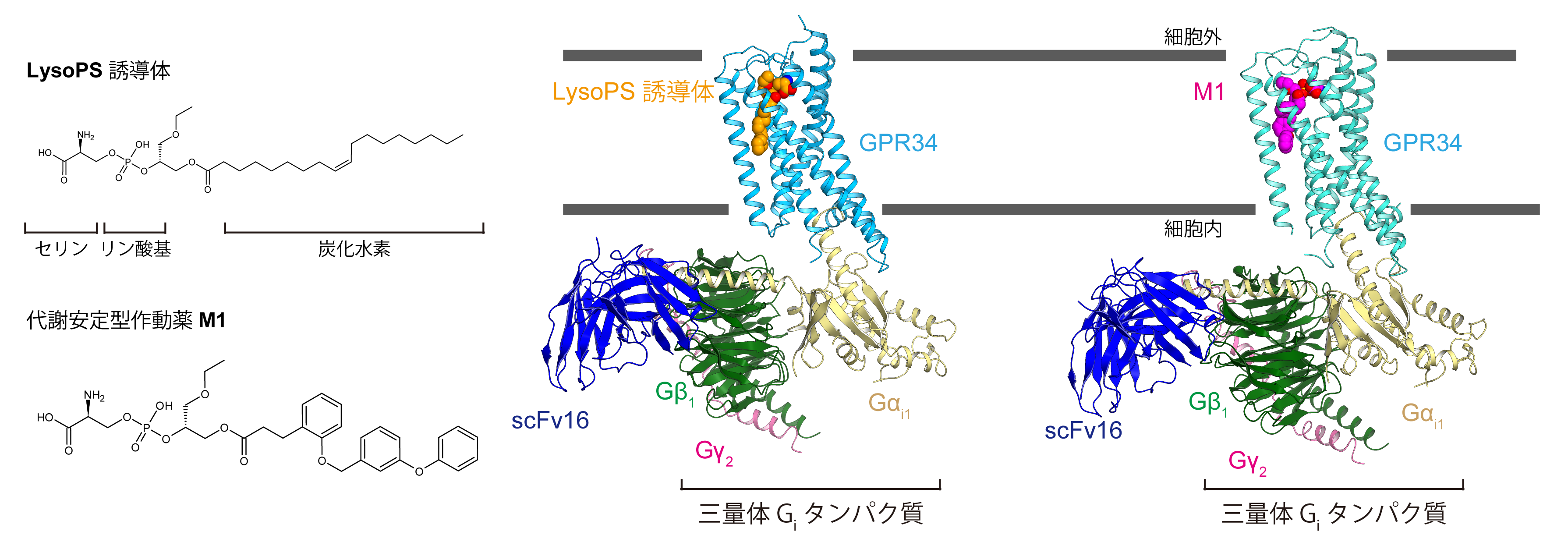
Figure: GPR34 agonists and GPR34-Gi complexes.
For more details, please read the article:
Tamaki Izume, Ryo Kawahara, Akiharu Uwamizu, Luying Chen, Shun Yaginuma, Jumpei Omi, Hiroki Kawana, Fengjue Hou, Fumiya K. Sano, Tatsuki Tanaka, Kazuhiro Kobayashi, Hiroyuki H. Okamoto, Yoshiaki Kise, Tomohiko Ohwada, Junken Aoki, Wataru Shihoya*, Osamu Nureki* (* responsible author)
2024. Structural basis for lysophosphatidylserine recognition by GPR34. Nature Communications. DOI: 10.1038/s41467-024-45046-z.


