Disclaimer: machine translated by DeepL which may contain errors.
Development of Solid Refrigerants Exhibiting the World's Highest Cooling Performance
Shinichi Okoshi, Professor, Department of Chemistry
Gaseous refrigerants are mainly used in cooling systems for air conditioners and refrigerators. However, new refrigerants called "solid refrigerants" are expected from the viewpoint of reducing environmental load. This is a system that controls the flow of heat in and out by inducing a solid-solid phase transition phenomenon through pressure stimulation. What kind of materials can be used as solid refrigerants with high performance? We have focused on Prussian blue analogues that exhibit a solid-solid phase transition and succeeded in developing a solid refrigerant with the world's highest cooling performance.
![]()
Currently, 20% of the electricity supplied by power plants is used to cool air conditioners and refrigerators, and cooling technology plays an important role in human activities. Conventional cooling technologies utilize a phase transition phenomenon between gases and liquids called gas refrigerants. Solid refrigerants are attracting attention from the viewpoint of Green Transformation (GX) and Sustainable Development Goals (SDGs), which aim to switch to renewable and clean energy for a decarbonized society.
In our laboratory, we have developed a number of metal complexes and metal oxides that exhibit various solid-solid phase transitions in response to external stimuli. In particular, we have observed phase transitions induced by light and electric fields in cyano-bridged metal complexes (-M-C≡N-M'-), which are called Prussian blue analogues. In this study, we focused on rubidium-cyanide-bridged manganese-iron-cobalt Prussian blueNote 1 and investigated the pressure-induced phase transition phenomena. The parent material, rubidium-cyanide-bridged manganese-iron Prussian blueNote 2, was first reported by Okoshi et al. in 2002. This substance has a three-dimensional network (-Fe(or Co)-C≡N-Mn-) in which the nitrogen atom of the cyano ligand coordinates to the manganese ion and the carbon atom coordinates to the iron (or cobalt) ion, and the rubidium ion is inserted into the gap between them. Rubidium ions are inserted in the gaps (left figure). The temperature dependence of the structure was investigated, and a phase transition due to charge transfer between the manganese and iron ions was observed. At room temperature, the charge state is in the MnII-NC-FeIII phase (high-temperature phase), but when the temperature is lowered, it changes to the MnIII-NC-FeII phase (low-temperature phase) at 192 K. When the temperature is increased from the low-temperature side, it returns to the original high-temperature phase from the low-temperature phase at 248 K. When the pressure effect of this material was investigated, it was observed that the phase transition temperature shifts significantly to the high-temperature side by the application of pressure. For example, the adiabatic cooling temperature at 560 MPa was -85 K, which is the highest cooling temperature in the world. For example, the adiabatic cooling temperature at 560 MPa was the world's highest cooling temperature of -85 K. This means that the cooling temperature was reduced to 3 °C when the pressure was released at 88 °C (center figure). The adiabatic cooling temperature at 340 MPa was -74 K. To experimentally verify these very large adiabatic cooling and heating temperatures, measurements were made using a thermocouple apparatus. As a result, a measured temperature increase of +44 K was observed when pressure (440 MPa) was applied, and a measured cooling temperature of -31 K was observed when pressure was released (right figure). These temperature changes are also the highest values in the world. In addition, the performance of the material did not deteriorate even after 100 cycles of repeated pressure application/release cycles. This research opens up new possibilities in the field of solid refrigerant materials and is expected to be a GX material that will make a significant contribution to future cooling technology.
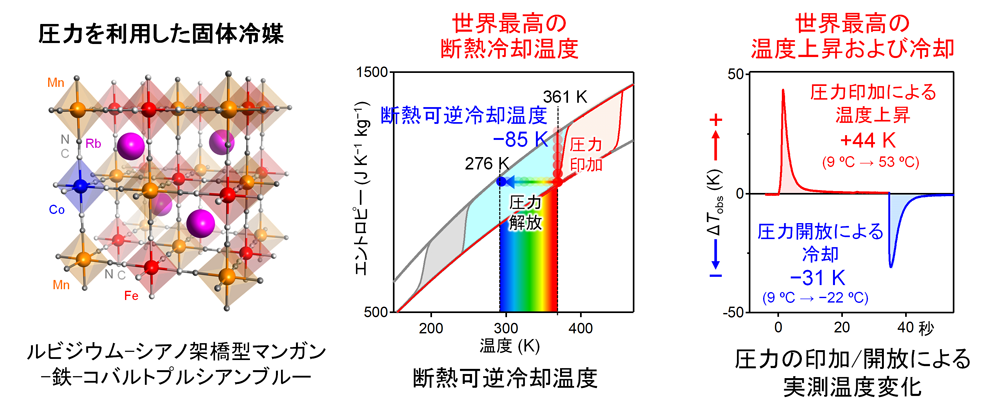 Giant adiabatic temperature change in rubidium-cyanide-bridged manganese-iron-cobalt Prussian blue (RbMn{[Fe(CN) 6]0.92 [Co(CN) 6]0.08}-0. 3H2O ). (Left panel) Crystal structure. (Middle) Adiabatic reversible temperature change. (Right) Directly observed temperature change (ΔTobs).
Giant adiabatic temperature change in rubidium-cyanide-bridged manganese-iron-cobalt Prussian blue (RbMn{[Fe(CN) 6]0.92 [Co(CN) 6]0.08}-0. 3H2O ). (Left panel) Crystal structure. (Middle) Adiabatic reversible temperature change. (Right) Directly observed temperature change (ΔTobs).Note 1: RbMn{[Fe(CN)6]0.92[Co(CN)6]0.08}-0.3H2O
Note 2: RbMn[Fe (CN)6]
This work was published in S. Ohkoshi et al. Nat. Commum. 14, 8466 (2023).
(Press release, December 27, 2023)
Published in The Rigakubu News, May 2024
Frontiers of Science for Undergraduates >


