Disclaimer: machine translated by DeepL which may contain errors.
Chemical Synthesis x Biological Synthesis for Next-Generation Manufacturing
Hiroki Ohguri, Professor, Department of Chemistry
Hiroki Ohguri, a professor in the Department of Chemistry, entered the field of research after training in chemical synthesis. Among the experimental sciences, it is a field in which physical and mental strength are said to be more important than intellectual power. In the laboratory of Professor Masahiro Hirama at Tohoku University, I worked on the artificial "chemical synthesis" of natural toxins with huge and complex structures. Using glucose as the starting material, I spent about 10 years with more than 10 colleagues until we succeeded in synthesizing the target molecule through more than 60 steps. Through a series of trial-and-error processes, I developed my skills as a researcher, and I gained the ability to somehow create the molecule that was the subject of my research. As a fledgling university faculty member, I have also come to understand that the ability to think about the behavior of molecules according to the logic of chemistry and to conduct a series of experiments in a timely manner is important for a "synthesist" to create the molecules he or she has envisioned at will.
Natural organic compounds (natural products), which express powerful and unique biological activities, have complex structures that are distinct from those of synthetic drugs. Why does nature go to the trouble of creating elaborate natural products? How can plants and fungi so easily assemble natural products with such strange and beautiful shapes? Naturally, I became interested in biosynthesis. For a "synthesist" who had been immersed in "chemical synthesis" until his early thirties, this was like the arrival of a black ship.
After studying in the U.S., I had an opportunity to work on "biosynthesis" in Prof. Hideaki Oikawa's laboratory at Hokkaido University from 2004. Although the chemical synthesis I worked on in Sendai and the biosynthesis I began studying in Sapporo have the same goal of providing natural products and their analogues, they have developed almost independently as different approaches. To begin with, enzymatic transformations that function in water and chemical transformations that use organic solvents have a water/oil relationship. It is not easy to link the two incompatible approaches to obtain complex molecules.
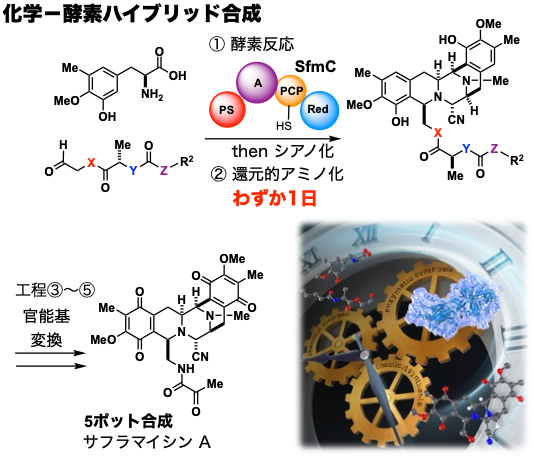
After becoming independent, I aimed to integrate "chemical synthesis" and "biological synthesis" based on my experiences in Sendai and Sapporo. This may be called a new challenge as a "synthesizer" with an aspiration to "synthesize" both. We have tackled the chemical-enzymatic hybrid synthesis of saframycin A, a natural product with anti-cancer activity, using biosynthetic enzymes as the target molecule. We succeeded in constructing a complex pentacyclic skeleton in only one day by enzymatic conversion of an organically synthesized substrate in aqueous solution, followed by rational chemical conversion of the generated unstable intermediate. Furthermore, they quickly synthesized saframycin A and its analogs through several transformations in a simple and easy manner.
By utilizing the advantages of "biosynthesis," in which elaborate enzyme-catalyzed reactions proceed continuously, and "chemical synthesis," in which substrates and intermediates can be freely modified, we can construct a material production platform that enables anyone to easily synthesize complex molecules. Currently, in addition to synthetic organic chemistry and synthetic biology, we are working on the developmental integration of structural biology and information science. In addition to discovering unused natural products that lie dormant on the earth, we hope to pursue infinite possibilities in next-generation manufacturing to freely create unexplored functional molecules with a low environmental impact.
Faculty of Science News, March 2023