Disclaimer: machine translated by DeepL which may contain errors.
Two catalysts working togetherContributing to a hydrogen society
Hiroyuki Miyamura, Assistant Professor , Department of ChemistryOsamu Kobayashi, Professor , Department of Chemistry |
![]()
In recent years, combined with efforts toward the SDGs and geopolitical risks, there has been an acceleration in the global movement to break away from dependence on fossil fuels and to build a carbon-recycling society. Hydrogen is a clean energy source compared to coal, petroleum, and natural gas, which emit large amounts of carbon dioxide, because hydrogen can extract energy by reacting with oxygen in the air, producing only water as a byproduct. However, energy production sites that can supply large quantities of hydrogen are often located far from Japan, where the hydrogen is consumed, making its transportation a challenge. In addition, since hydrogen is a gas, it is inefficient to transport it in its original form. Although the volume can be significantly reduced by cooling it to liquid form, a large amount of energy is required for cooling, and new tankers and storage facilities need to be built specifically for the storage and transportation of hydrogen. Under these circumstances, the organic hydride method, in which hydrogen is chemically reacted with aromatic compounds such as benzene and toluene to form organic hydrides, which are then transported as "hydrogen storage" so to speak, is attracting attention. Benzene and toluene, which are cyclic unsaturated compounds, react with three molecules of hydrogen under the presence of a catalyst to convert them into cyclohexane and methylcyclohexane, which are liquid organic hydrides at room temperature and pressure. In other words, one aromatic compound can store three molecules of hydrogen. Since these organic hydrides are also components of gasoline, they have the advantage of utilizing existing infrastructure such as tankers and storage facilities.
Thus, catalytic hydrogenation of aromatic compounds can be applied to hydrogen storage and transport using the organic hydride process, and is an important reaction for the realization of a hydrogen society and for the synthesis of high value-added chemicals such as pharmaceuticals. However, hydrogenation of aromatic compounds with bulky or electron-rich substituents is difficult and requires harsh reaction conditions such as high temperature and high pressure, and the development of an efficient synthetic method has been a challenge.
 |
|
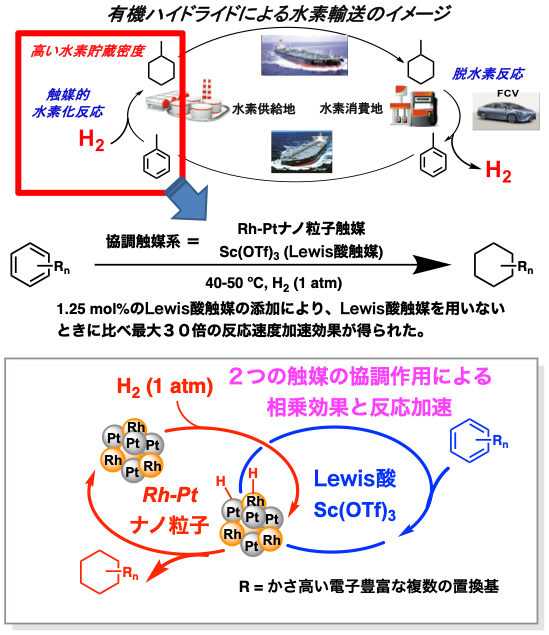
| Figure: Hydrogenation of aromatic compounds using a catalytic system consisting of Rh-Pt nanoparticle catalysts and Lewis acid catalysts | |
In the present study, we focused on a new type of catalytic system in which multiple catalysts can synergistically reduce the activation energy of the transition state. We found that aromatic compounds, which were difficult to hydrogenate, were smoothly hydrogenated at 1 atm hydrogen and low temperatures (below 50 ºC) by the cooperative effect of heterogeneous rhodium-platinum nanoparticles and Lewis acid scandium catalysts.
In the future, this catalytic system is expected to contribute to the achievement of the SDGs by realizing resource and energy savings in the synthesis of pharmaceuticals and other chemical products by developing a continuous flow process that is closer to practical use. The successful development of the catalytic reaction has also paved the way for the development of various aromatic compounds, which have been difficult to hydrogenate, as new hydrogen carriers for hydrogen transport.
These research results were published in H. Miyamura and S. Kobayashi, Angewandte Chemie and Angewandte Chemie International Edition e202201203 (2022).
(Press release on April 15, 2022)
Published in Faculty of Science News July 2022
Communicating to Faculty Research Students on the Frontiers of Research>
*August 26, 2022, additional authors were added and revised.


