Disclaimer: machine translated by DeepL which may contain errors.
Nuclear Order and Existence Limits Probed by Mass
Shinichiro Michimasa( Assistant Professor, Center for Nuclear Study) |


All the matter that surrounds us is composed of atoms, and the nucleus, which is composed of protons and neutrons, occupies the center of the matter. In nuclei, there are isotopes of the same element with different numbers of neutrons, and nuclei with the same number of protons and neutrons are generally more stable. As the number of neutrons increases in a nucleus, the ability to bind neutrons gradually weakens. As the number of neutrons in the nucleus increases, the binding force is lost, and this is the limit of existence of the nucleus.
The binding force of neutrons in a nucleus is conveniently indexed by the "two-neutron separation energy," which is calculated from the mass difference with an isotope containing two fewer neutrons. The figure shows the variation of the experimentally measured two-neutron separation energies of calcium (Ca), scandium (Sc), titanium (Ti) and vanadium (V) with neutron number. The nuclei whose nuclear masses were measured in this study have a lifetime of about 10 ms, which is the first data obtained by our measurement technique, which is fast and has a mass accuracy of 6 significant digits. The figure clearly shows that the two-neutron separation energy decreases as the number of neutrons increases. However, the decrease is not monotonous, and there is a clear structure.
First, we focus on Ca and Sc isotopes. The large decrease in the Ca isotope indicates a large energy gap between neutron levels, suggesting the onset of magic properties at neutron number 34. Although nuclear structure theory predicting magic properties in Ca nuclei with a neutron number of 34 has already been reported, this is the first observation of the sudden appearance of a new order with a magic number of 34 in nuclear neutrons with a decrease of one proton from Sc to Ca.
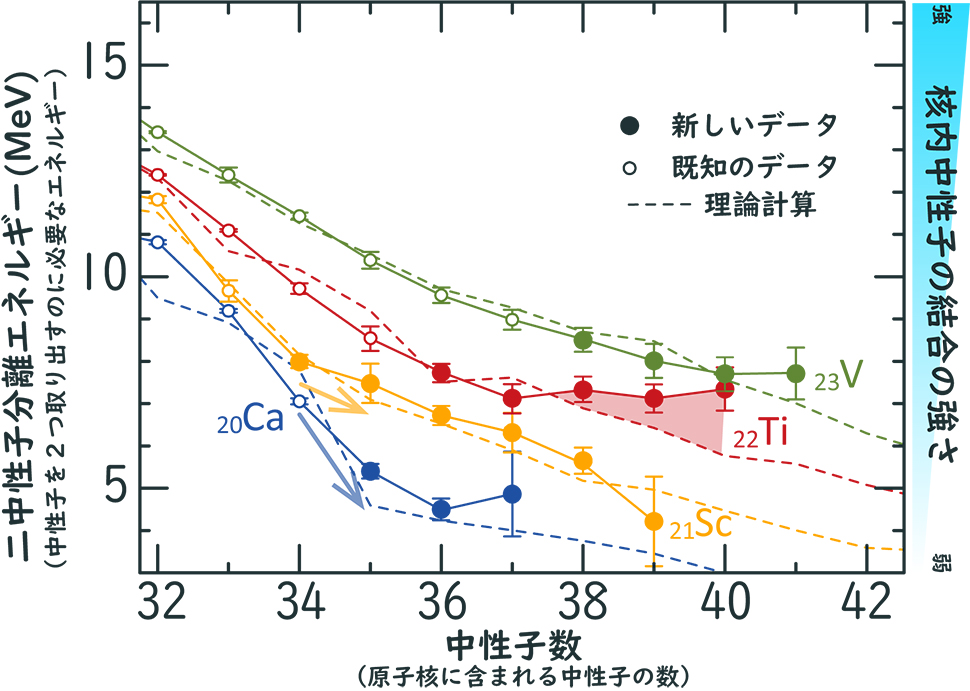 |
||
| Bi-neutron separation energies of calcium (Ca), scandium (Sc), titanium (Ti), and vanadium (V) isotopes near the existence limit. The solid lines connect the isotopes and show their variation with neutron number. The dashed line is the theoretical value.
|
Next, let us look at the Ti and V isotopes around the neutron number of 40. Here, too, there are differences among the isotopes. The di-neutron separation energy of the Ti isotope does not decrease with increasing neutrons. Compared to the latest theoretical calculations (dashed line in the figure), the binding strength with neutrons is clearly stronger. These facts indicate that in Ti isotopes with high neutron abundance, new structural changes of nucleons in the nucleus occur, maintaining the binding strength and stabilizing the nucleus as a whole. At present, the detailed mechanism of this stabilization is not known. However, the discovery of stabilization phenomena occurring in neutron-rich nuclei suggests that the number of possible nuclear species may be larger than previously expected.
Through the results of this research, we have witnessed a new order in nuclei composed of two types of particles, protons and neutrons. In research, new questions are always the driving force for deeper understanding. The observation of nuclear mass change must be one of the paths to a deeper understanding of the origin of the enigmatic matter that is the nucleus.
This study is based on the work of S. Michimasa et al . , Phys. Rev. Lett . 121, 122506 (2018) and Phys. Rev. Lett . 125, 122501 (2020).
(Press release, September 16, 2020)
Published in the January 2021 issue of Faculty of Science News
Communicating to Faculty Research Students on the Frontiers of Research>


