Disclaimer: machine translated by DeepL which may contain errors.
The Rigakubu News
The Rigakubu News, January 2025.
The Frontiers of Research for Undergraduates >
Photoreaction Creates Drug Candidate Molecules with Complex Structures
Hiroki Ohguri, Professor, Department of Chemistry
Opioid analgesics for pain relief have contributed greatly to the development of medicine, but have also caused a serious social problem of drug addiction.
Recently, a plant-derived molecule (natural product) called iboga alkaloid has attracted attention as a candidate for the treatment of drug addiction.
In this study, we developed an innovative method for the efficient synthesis of complex and beautiful molecular skeletons of iboga alkaloids.
By designing intermediates with diverse reactivity and precisely controlling their reactivity with light, we succeeded in creating
three molecular skeletons.
The research is expected to lead to new developments in life science research and drug discovery research utilizing natural products and their analogues, which have been difficult to synthesize chemically.
![]()
Natural products found in nature have contributed greatly to the development of innovative drugs. Iboga alkaloids, which are biosynthesized by plants, have a pentacyclic skeleton that incorporates the structure of serotonin, a neurotransmitter. The side chain of serotonin ( -CH2CH2NH2 ) is flexible and can rotate freely. In contrast, in the natural product ibogaine, a typical iboga alkaloid, the side chain is fixed and the entire molecule forms a rigid polycyclic structure. This structure allows ibogaine to bind to receptors in vivo in a complementary and tight manner, and to modulate intracellular signal transduction through the receptors.
These properties make iboga alkaloids very promising motifs for nature-inspired drug development, and their complex and beautiful molecular structures have attracted researchers in the field of synthetic chemistry for more than 60 years. The author and his colleagues are engaged in research to mimic the process by which plants biosynthesize natural products through multi-step enzymatic reactions, while creating a diverse group of compounds that transcend the framework of such reactions. Specifically, they are pursuing methods to efficiently synthesize natural products and their analogues by rationally modifying short-lived virtual biosynthetic intermediates1 and designing stabilized pluripotent intermediates2.
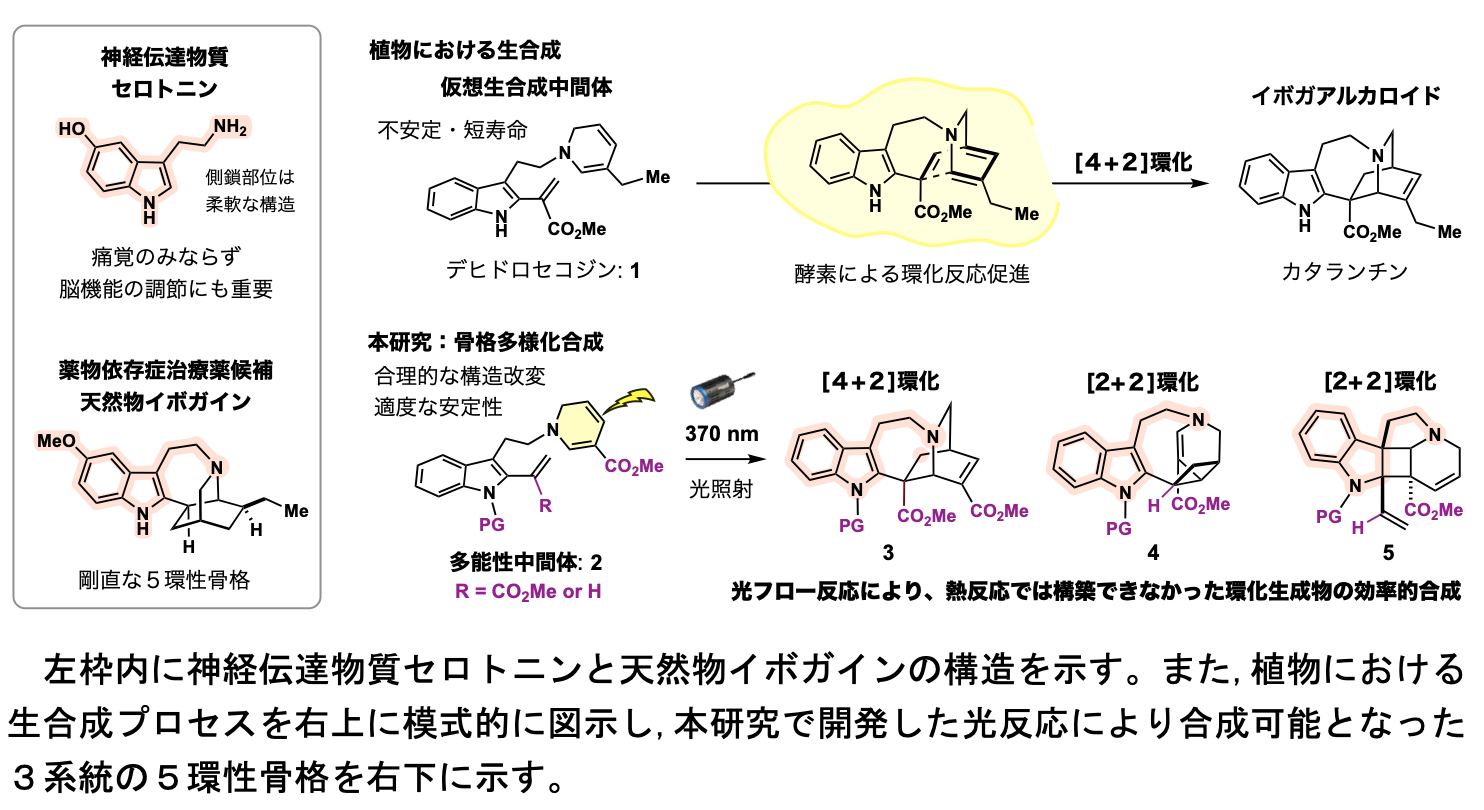 The structures of the neurotransmitter serotonin and the natural product ibogaine are shown in the left frame. The biosynthetic process in plants is schematically illustrated in the upper right corner, and the pentacyclic skeleton of the three strains developed in this Research Student is shown in the lower right corner.
The structures of the neurotransmitter serotonin and the natural product ibogaine are shown in the left frame. The biosynthetic process in plants is schematically illustrated in the upper right corner, and the pentacyclic skeleton of the three strains developed in this Research Student is shown in the lower right corner.In the biosynthesis of iboga alkaloids, the key reaction is the formation of a six-membered ring by intramolecular cyclization of [4+2]-type dehydrosecodine 1, a virtual intermediate. In general, this [4+2]-type cyclization reaction proceeds by thermal energy, but in this study, we developed an unprecedented approach in which the cyclization is activated by light. The [4+2]-type cyclization reaction using intermediate 2 as a substrate was difficult to carry out under conventional heating conditions, but we found that the reaction proceeds efficiently under LED lamp irradiation, and we succeeded in rapidly synthesizing the pentacyclic skeleton 3. To prevent the highly reactive 2 and product 3 from decomposing under photoreaction conditions, they employed a light-flow synthesis technique in which a solution of substrate 2 is continuously fed into the photoreactor and the reaction is completed within 25 minutes. As a result, the efficiency of synthesis of the target pentacyclic skeleton 3 was greatly improved compared to the conventional method.
Furthermore, by modifying the structure of the pluripotent intermediate 2, the reaction to form a 4-membered ring by [2+2]-type cyclization was preferentially promoted, and the synthesis of 4 and 5, which formed different 5-ring skeletons, was successfully achieved. The three pentacyclic skeletal groups 3-5 synthesized in this study have three-dimensional structures in which the side chain of the neurotransmitter serotonin is immobilized in different ways. Thus, we were able to create a group of molecules that mimic the structure and function of bioactive natural products while extending their framework. This method has opened the way for the rapid and flexible synthesis of iboga alkaloid analogues, which are not only candidates for the treatment of opioid addiction but also promising drugs for the treatment of cancer. This research is expected to lead to new developments in life science research and drug discovery research based on natural products.
The results of this study were published in G. Tay et al., Chem. Sci., 15, 15599 (2024).
(Press release on September 19, 2024)


