Disclaimer: machine translated by DeepL which may contain errors.
Entropy Revisited
Eiichi Nakamura (Department of Chemistry, University Professor / The University of Tokyo, Emeritus Professor)
Takayuki Nakamuro (Department of Chemistry, Project Associate Professor)
Entropy (S) is a thermodynamic physical quantity defined by Rudolf Julius Emmanuel Clausius in 1865 as "the value of heat quantity (Q) divided by temperature (T) (S = Q / T ). In 1877, Ludwig Eduard Boltzmann interpreted entropy as S = kB ln (Ω) from the framework of statistical mechanics using the microscopic number of states (Ω). Since S increases as Ω increases, entropy is a measure of clutter. However, how to obtain Ω experimentally was a difficult problem. We discovered a method to determine the entropy change, ΔSd, from the probability of crystal disorganization caused by electron irradiation.
![]()
In high school chemistry, students are taught that "entropy is a measure of molecular disorder," but this is not clear from Clausius' definition of S = Q/T. This is because, as we have seen, the entropy is a measure of the disorder of a molecule, not the disorder of a crystal. That is because Clausius did not believe that matter was composed of atoms or molecules. Boltzmann's S = kBln (Ω) is the measure of clutter, but how to measure Ω has been a longstanding problem. Over a period of five years starting in 2019, we discovered that the entropy change ΔSf associated with crystal melting is consistent with the physical quantity determined from the decay rate of the crystal diffraction signal by electron beams (Figure a), which we named ΔSd. ΔSd = ΔSf for various crystals as illustrated in Figure b. This method of determining ΔSd using samples with femtogram quantities (10-15 g) or less is expected to lead to innovations in a wide range of fields, including chemistry, materials, and structural biology.
As is typical when a new research field develops, this research began with a question unrelated to entropy: Since one of the authors (Nakamura) captured an image of the movement of a single organic molecule by transmission electron microscopy in 2007, it has been believed to this day that "fast electrons smash organic molecules, so why should Nakamura's research be somehow wrong? Therefore, something is wrong with Nakamura's research. Nakamura's research has been criticized constantly. In the spring of 2019, we decided to ask Dongxin Liu, a first-year master's student at the time, to work with Nakamuro to investigate the mechanism of decomposition of organic molecules by electron beams. This is Nakamuro's first work in his second year at the University of Tokyo.
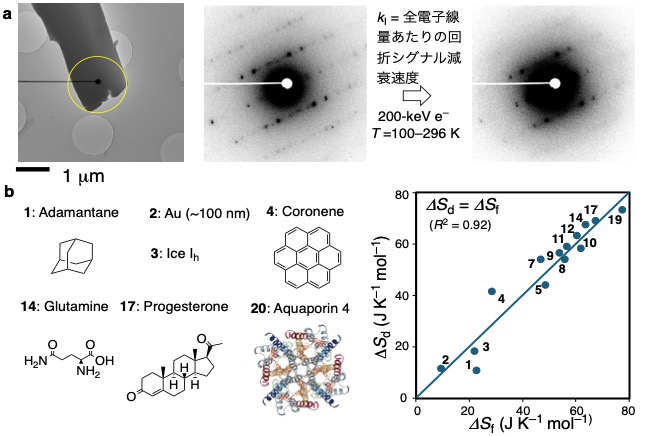 The outline of this study: a) Quantification of the decay of the electron diffraction signal of microcrystals. b) ΔSd= ΔSffor various crystals as illustrated here.
The outline of this study: a) Quantification of the decay of the electron diffraction signal of microcrystals. b) ΔSd= ΔSffor various crystals as illustrated here.By exposing the various crystals to electron beams and measuring the temperature dependence of the rate of decay of the diffraction signal, it was found that the rate constant depends almost exclusively on the frequency factor A in the Arrhenius equation, regardless of the crystal type. The organic molecules do not decompose, but only undergo a conformational change. The conventional wisdom is wrong. I was immediately convinced. The difficult part was to elucidate the meaning of frequency factor A. Without the help of my colleague, Professor Kaoru Yamauchi, it would have been difficult to reach the correct answer.
At first, ln(A) seemed to correlate with the enthalpy of melting ΔHf (reported by Dongxin Liu on April 18, 2020). Upon further investigation, we found that ln (A) and melting entropy ΔSf are strongly correlated via the inverse of the gas constant R (May 2, 2020), and we found the equation ln (A) = aΔSf /R+ ln(AINT ). At first, the meaning of ln(AINT ) was unclear and the value of a could not be determined. a = 1.00 was determined on April 11, 2024, three days before I met with the editor of Science to finalize the adoption of the formula, and I became convinced that AINT was the scattering cross section of electrons involved in molecular disorganization. This was during the writing of this paper. The research is continuing.
The results of this study were published in D. Liu et al., Science, 384, 1212(2024)
Published in The Rigakubu News, September 2024
The Frontiers of Research for Undergraduates >


