DATE2024.05.31 #Press Releases
Quantifying Entropy Changes from the Disorder of Individual Molecules
Measuring Melting Entropy at the Nanoscale Using Statistical Mechanics Techniques
Summary
In the significant curriculum revision for high school in Japan chemistry from 2023, entropy is introduced as a measure of "disorder," a crucial shift in teaching concepts like the melting of ice into water. Despite Ludwig Boltzmann linking entropy to the number of microscopic states back in 1877, defining these states precisely has long challenged researchers and educators. Professor Eiichi Nakamura's team at the University of Tokyo has demonstrated that changes over time in the electron diffraction signals of crystals can reflect the variety of molecular structures within, corresponding to changes in Boltzmann's microscopic states—thus entropy. This innovative method, using minuscule amounts of materials from organic semiconductors to proteins, allows for entropy measurement on the nanoscale, diverging from traditional methods that require large quantities and opening new possibilities in nanoscience.
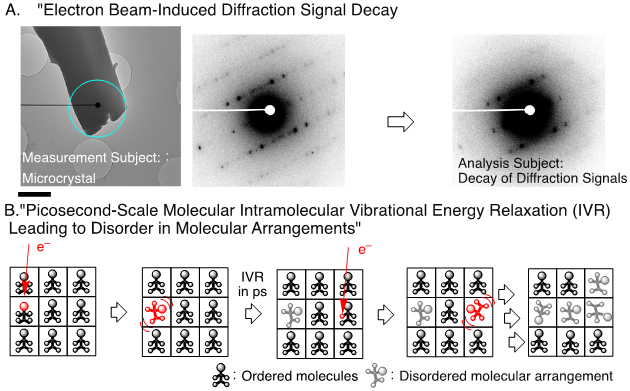
Figure : Conceptual Diagram of the Study. (A) Electron beams are directed at the light blue area to capture diffraction signals. (B) The attenuation of diffraction signals is attributed to the disorganization of molecular arrangements due to interactions between electrons and molecules. The scale bar in the diagram represents one micrometer. The decay rate of electron diffraction signals during 200 keV electron beam exposure was kinetically analyzed across temperatures ranging from 100 to 296 K.
Journals
-
Journal name Science Title of paper


