Disclaimer: machine translated by DeepL which may contain errors.
Keep your guess protein molecules shining in your cells
Sawako Enoki (Project Assistant Professor, Universal Biology Institute)
Yasushi Okada (Professor, Graduate School of Medicine / Adjunct Professor, Department of Physics)
With the development of microscopy technology, even a single molecule can be directly observed by binding a fluorescent dye to the target molecule. This technique is the basis of super-resolution fluorescence microscopy.
However, under such observation conditions, conventional fluorescent dyes break down and cease to shine (fade) in a few seconds. This makes it difficult to continue observing the function of the molecule of interest or to observe it over time using super-resolution fluorescence microscopy.
We have developed a new fluorescent dye that does not fade easily and are applying it to the observation of living cells.
![]()
Fluorescent dye molecules can be seen with the naked eye using a fluorescence microscope. The fluorescent dye molecules glow with the same brightness as stars in the night sky, but fade away in a few seconds. The fluorescent dye breaks down and ceases to glow due to a phenomenon called fading. Experiments in which we continue to look at molecules are limited by the fading.
Fluorescent dyes are organic compounds with conjugated double bonds such as benzene rings. The introduction of elements other than C, H, O, and N changes the properties of the fluorescent dye. Dr. Yamaguchi, Dr. Taki, and their colleagues at Nagoya University have developed a new fluorescent dye, PREX710, by introducing a highly electron-withdrawing atomic group (phosphine oxide P=O) containing a phosphorus element (Figure 1(a)). We tried it and, to our surprise, it continued to glow for more than several minutes under the above conditions, an improvement of nearly 100-fold.
However, PREX710 is a positively charged molecule with low cell membrane permeability, making it unsuitable for intracellular experiments. In this study, a 2-carboxy-benzo(b)thiophen-3-yl (BT) group was introduced into the P=O-incorporated phosphorhodamine (POR) fluorescent dye backbone to improve cell membrane permeability (Figure (c), red portion). The ring-closed structure with no charge is expected to improve cell membrane permeability (right side of Fig. (c)). At the other end of the POR, chloroalkane was introduced as a ligand to bind specifically to the target protein in the cell (Fig. (c) blue, Halo-tag, Promega).
 (a) Structural formula of the previously developed PREX710. The positive charge makes the film less permeable.
(a) Structural formula of the previously developed PREX710. The positive charge makes the film less permeable.(b) Silicon rhodamine with silicon Si. Its use is increasing due to its high fading resistance.
(c) This study. (c) Equilibrium structure between the ring-closed structure without charge (right) and the highly fluorescent open-ring structure (left).
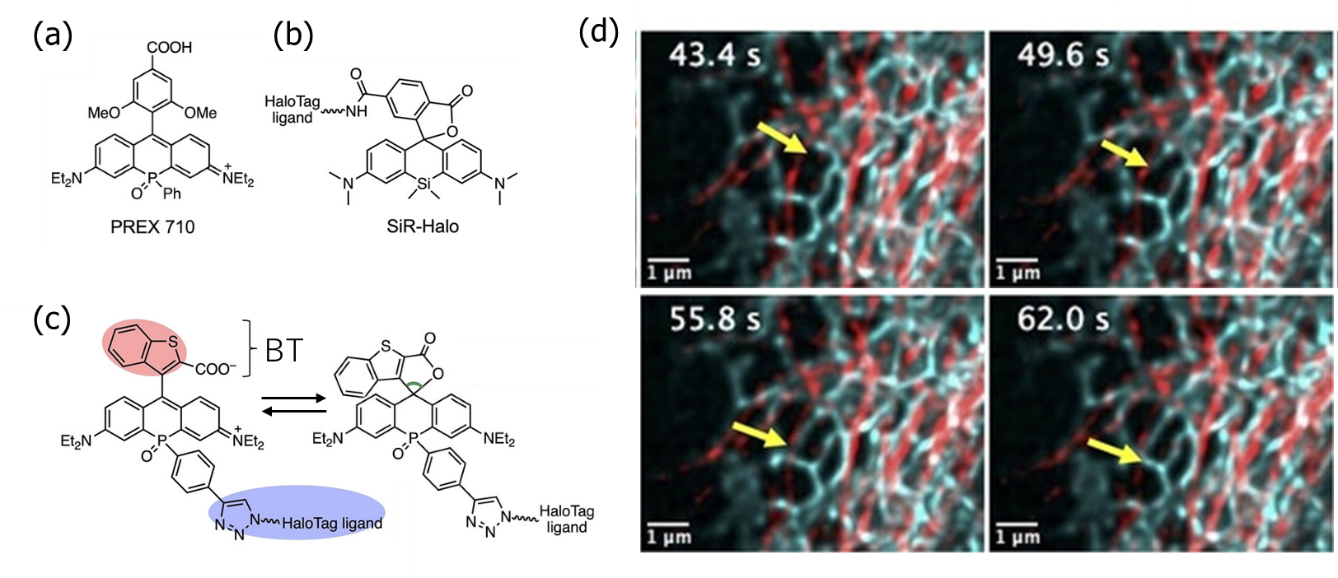
(d) Intracellularly, microtubules were stained with SiR (red) and ERs with transPOR (cyan). super-resolution microscopy for more than 1 min did not fade, and dynamics such as ER tube extension (arrow) was observed.
BT and POR are orthogonally arranged due to steric hindrance, resulting in cis- and trans-isomers in which the carboxy group of BT and the PO of POR face the same direction. cis-isomers easily form aggregates in the lipid bilayer and stain intracellular membrane systems nonspecifically. On the other hand, the trans isomers suppressed aggregate formation in the lipid bilayer and were able to pass through the cell membrane to label the target protein intracellularly. The fact that the permeability of the cell membrane differs greatly depending on the steric structure is an unexpected result, and is a new finding that will serve as a reference for future development.
A similar idea has been applied to silicon rhodamine (SiR), in which silicon Si is introduced as a fluorescent dye with high fade resistance (Figure (b)). Since trans POR has a longer wavelength than SiR, it can be combined with SiR to enable super-resolution fluorescence microscopy with double staining over time (Figure (d)). In the process, it was also confirmed that SiR faded first and trans POR was 3 to 4 times more difficult to fade than SiR.
This study is a collaboration of chemists (Prof. Shigehiro Yamaguchi and Project Associate Professor Masayasu Taki at Nagoya University), computational scientists (Prof. Florence Tama at RIKEN, molecular dynamics simulations), and us (biophysics, microscope development). Through such interdisciplinary collaborations, we hope to continue to push the boundaries of conventional research and challenge the goal of "continuing to see how a guess protein molecule works in the cell.
The results of this research have been published in Q. Wu et al, Angewandte Chemie International Edition 63, e202400711 (2024).
Published in The Rigakubu News July 2024
The Frontiers of Research for Undergraduates >


