Disclaimer: machine translated by DeepL which may contain errors.
New Combination Opens the Door to "Manufacturing in Chemistry"
Mitsuhiko Shionoya, Professor, Department of Chemistry
The periodic table is the starting point of the "manufacturing of chemistry. In conventional organic synthesis, atoms are connected by strong covalent bonds such as carbon-carbon bonds, and new molecules have been created one after another. Although the author learned the "ABCs" of organic synthesis in the Department of Pharmaceutical Chemistry in the Faculty of Pharmaceutical Sciences, he felt that the viewpoint of synthetic chemists was changing with the concept of "supramolecules," which was proposed about 50 years ago. This trend provided an opportunity for the author to use the periodic table more widely. Supramolecules are molecular assemblies consisting of two or more molecules loosely connected by relatively weak bonds other than covalent bonds (e.g., hydrogen bonds) or hydrophobic effects. Since the intermolecular bonds are weak, they can be broken or interchanged by external stimuli (temperature or light). Therefore, not only do they change in shape and size, but also cooperative interactions between molecules can give rise to properties not found in individual molecules. Furthermore, since metal atoms can also be used as materials for supramolecular synthesis, supramolecular formation accompanied by changes in structure and properties unique to metals has attracted the attention of many researchers. This concept of supramolecular chemistry has developed synthetic chemistry, which skillfully deals with strong molecular bonds and loose connections between molecules, and has created new academic values linking different fields.
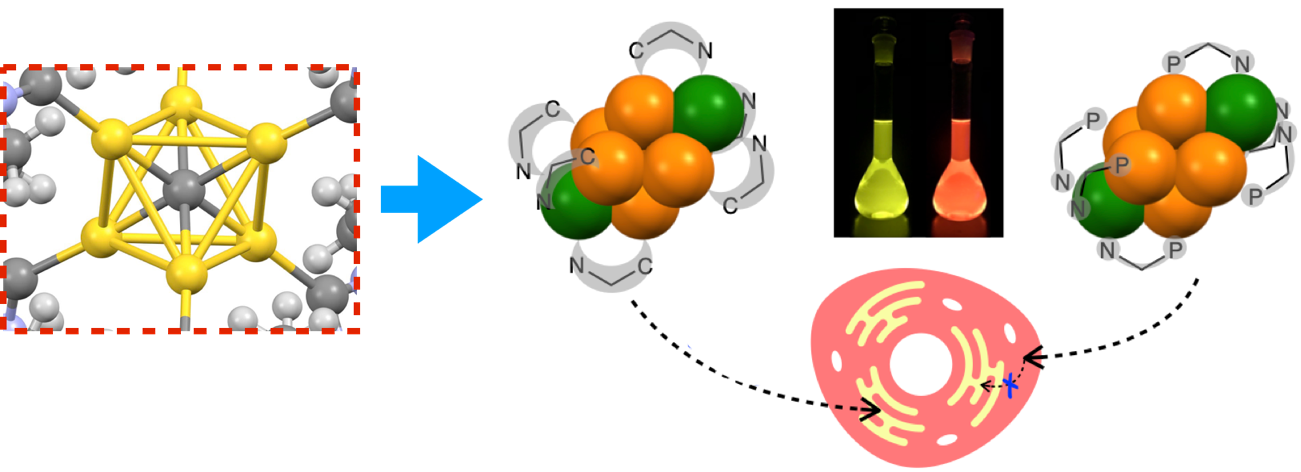
In 2018, the author reported a carbon-centered gold(I) cluster ([CAu6L6]2+) with an N-heterocyclic carbene ligand (L). The central carbon ion (C4-) of this Au(I) cluster is bonded to six Au(I) ions (Au+), and one ligand (L) is attached to each of these Au(I) ions from the outside. This molecular structure is stabilized not only by the 12 C-Au bonds but also by a large number of Au-Au interactions. That is, each Au(I) ion is bound to two carbon atoms, which in turn interact with four Au(I) ions. The molecule is weakly luminescent in solid, but not in liquid (Figure left).
 |
|||
| Carbon-centered gold(I) cluster (left) and gold(I)-silver(I) cluster glowing and moving in a cell | |||
Based on these results, the author (Supramolecular Chemistry) would like to "make glowing chameleon molecules," Dr. Masahiro Ehara (Theoretical Chemistry @ Institute for Molecular Science) would like to "reveal the individuality of glowing molecules," Dr. Takemasa Ozawa (Analytical Chemistry @ University of Tokyo) would like to "light up biological molecules with glowing molecules," and Dr. Toshiaki Gamaike (Analytical Chemistry @ University of Tokyo) would like to "pursue molecules that run around glowing. (Analytical Chemistry @ Tokyo Institute of Technology), and Dr. Toshiaki Kabaike (Bioinorganic Chemistry @ Tokyo Institute of Technology), who wanted to "pursue molecules that run around while glowing! Finally, they discovered that gold(I)-silver(I) clusters emit strong phosphorescence and move along a fixed path in the cell toward the center (Figure right: Nature Communications, 13, 4288, 2022). No one knows yet whether it acts alone or has a partner.
The carbon and gold(I) ions in the gold(I) cluster have a somewhat unusual structure, with more hands than usual. Carbon, which normally has four bonding hands in organic compounds, has six hands, while gold(I), which has two hands, has six hands. Theoretical calculations show that each bond is weaker than a covalent bond by the number of hands, but the whole molecule is stabilized by being connected by many hands. This provides a new guideline for the approach of building a molecule by appropriately arranging bonds of different strengths. The interdisciplinary research is opening a new frontier in the "manufacturing of chemistry.
The Rigaku-bu News, January 2024


