Disclaimer: machine translated by DeepL which may contain errors.
Direct Conversion of Phase Transition Energy of Polymers into Electricity
Huangfang Zhou (Project Assistant Professor, Department of Chemistry)
Teppei Yamada, Professor, Department of Chemistry
Gibbs energy, which we learn in thermodynamics, is related to "free energy" that can be converted into useful work.
The Gibbs energy change (heat of combustion) in the production of CO2 and water from oil and oxygen can be used to turn a
turbine to produce electricity.
Fuel cells can convert this reaction energy directly into electricity.
Then, what kind of free energy can be directly converted into electric power?
How about the melting energy of ice?
We have demonstrated for the first time in the world that certain types of phase transition energy can be directly converted into electric power.
![]()
Electricity is a convenient energy source, and it needs to be produced with as little CO2 emissions as possible. Power generation using renewable energy sources such as solar power, geothermal power, tidal power, and waste heat is an important issue for the 21st century. In addition to semiconductor-based methods for converting heat into electricity, research on thermochemical batteries that use oxidation-reduction reactions has been attracting attention in recent years.
A thermochemical battery is a thermoelectric conversion system that uses a redox reaction. When a substance that exhibits a redox reaction is dissolved in a solution, an equilibrium is created in which the oxidized and reduced substances exchange electrons with each other. This equilibrium, like other equilibria, shifts depending on changes in various conditions, and the equilibrium shifts also occur depending on temperature. Therefore, if an electrode is inserted into each solution at different temperatures, the equilibrium shifts between the low and high temperature sides, and a current in one direction is generated at the electrode. This is the principle of the thermochemical battery. We believe that this thermochemical battery system can be used as a basis for energy conversion. To demonstrate this, we used the phase transition of polymers.
The polymer shown in Figure (a) undergoes a phase transition from a helical string structure (coil structure) at temperatures below 30 °C to a rounded structure (globule structure) at higher temperatures. The coiled structure is hydrophilic and the molecules disperse in water, whereas the globule structure is hydrophobic and the macromolecules aggregate. This can be regarded as a kind of phase transition, whereby heat is absorbed in the same way that ice becomes water.
We have introduced a redox-enabled moiety into this macromolecule. When oxidized, the molecule becomes more charged and hydrophilic, thus becoming a coil, and when reduced, it conversely becomes a globule. In other words, we have created a compound that can electrically undergo a coil-globule phase transition. This coil-globule transition should result in heat generation on the oxidation side and cooling on the reduction side. When electricity was actually applied, a cooling effect was observed (c). This is an electron cooling device based on a new mechanism.
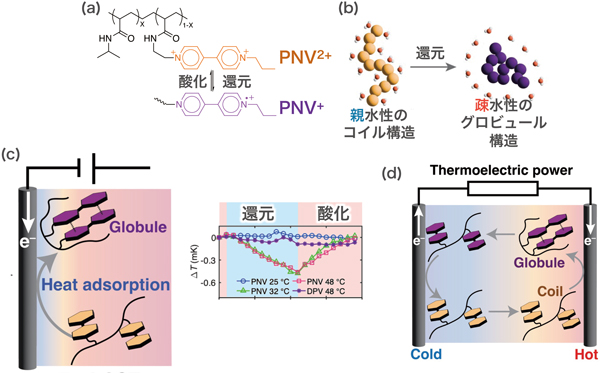 (a) Schematic diagram of PNV. The polymer in the main chain shows the coil-globule transition, and the viologen moiety in the side chain shows redox ability. (b) Schematic of the coil-globule transition. (c) When PNV is reduced by an external current, the polymer curls up and absorbs heat. No heat absorption is observed at 25 °C, where the coil-globule transition does not occur. (d) Depending on the external temperature difference, the polymer is rounded and reduced at the high temperature side, while it is stretched and oxidized at the low temperature side, thus generating electricity.
(a) Schematic diagram of PNV. The polymer in the main chain shows the coil-globule transition, and the viologen moiety in the side chain shows redox ability. (b) Schematic of the coil-globule transition. (c) When PNV is reduced by an external current, the polymer curls up and absorbs heat. No heat absorption is observed at 25 °C, where the coil-globule transition does not occur. (d) Depending on the external temperature difference, the polymer is rounded and reduced at the high temperature side, while it is stretched and oxidized at the low temperature side, thus generating electricity.
Furthermore, when the compound is subjected to a temperature difference, the oxidation state, in which the coiled state is stable at low temperatures, and the reduction state, in which the globule state is stable at high temperatures, occur. This gives the voltage (or more precisely, the voltage change per unit temperature difference, Seebeck coefficient) of thermoelectric conversion (d). It is also clear that the voltage per unit temperature difference (V/K) obtained in this power generation is almost identical to the entropy change of the phase transition latent heat per charge (J/K/C) during the phase transition by the redox reaction. This means that we have succeeded in directly converting the Gibbs energy change produced by the phase transition into electrical energy. In other words, we have succeeded in developing a new method for converting the Gibbs energy of phase transitions into electrical energy. This phenomenon is expected to be applicable not only to the coil-globule transition of polymers, but also to all free energies that are changed by redox reactions.
This study was published in T. Yamada et al. Advanced Materials, 35, 2303341 (2023).
(Press release, July 18, 2023)
The Rigaku-bu News, November 2023
Communicating to Faculty Research Students on the Frontiers of Research>


