DATE2022.08.10 #Press Releases
Gold and silver nanoclusters that emit phosphorescence in cells!
Disclaimer: machine translated by DeepL which may contain errors.
Zhen Lei (Project Assistant Professor, Department of Chemistry)
Mizuki Endo, Assistant Professor, Department of Chemistry
Hitoshi Ube (Assistant Professor, Department of Chemistry)
Masahiro Ehara (Professor, Institute for Molecular Science)
Takemasa Ozawa, Professor, Department of Chemistry
Prof. Mitsuhiko Shiotani (Department of Chemistry)
Key points of the presentation
- We found that carbon-centered gold-silver ( CAuI6AgI2 ) clusters with nitrogen-containing heterocyclic carbene (NHC) ligands emit strong phosphorescence in living cells at room temperature and are transported in a structure-specific manner.
- Precise design of the ligand of the carbon-centered gold ( CAuI6 ) cluster and the addition of silver ions enabled the enhancement of the efficiency of phospholuminescence and tunability of the wavelength, thereby controlling the cellular uptake pathway and localization of the cluster in cell organelles.
- This study demonstrates that multinuclear metal ion clusters consisting of core/shell ligands and metal ions can make a significant contribution to the development of photo-bioanalysis as a group of strongly phosphorescent molecules.
Summary of Presentation
Luminescent metal nanoclusters are expected to exhibit unique physical properties in the cluster structure depending on the ligand structure, metal type, and number and arrangement of nuclei. In this study, Professor Mitsuhiko Shiotani and Professor Takemasa Ozawa at Graduate School of Science, The University of Tokyo, Professor Masahiro Ehara at Research Center for Computational Science and Institute for Molecular Science, National Institutes of Natural Sciences, and Professor Toshiaki Kamaike at School of Bioscience and Biotechnology, The University of Tokyo, and their colleagues have developed carbon-centered gold-silver ( CAuI6AgI2) clusters designed and synthesized with a carbon-centered gold-silver (CAuI6) ( AgI2) ligand and found that these molecules emit strong phosphorescent (Note 2 ) light in solution, and theoretical calculations have revealed that the NHC ligand contributes to the phosphorescent emission. When these phosphorescent gold-silver clusters with long luminescence lifetime were used for cell imaging, it was found that they selectively localize to the pathway of cellular uptake and to specific organelles, confirming their superior function, which is different from the non-selective uptake of conventional phosphine ligands. This study demonstrated that multinuclear metal ion clusters consisting of highly designable ligands and metal ions can contribute significantly to the development of photo-bioanalysis as a new group of strongly phosphorescent materials (Figure 1).
The research results have been published online in Nature Communications.
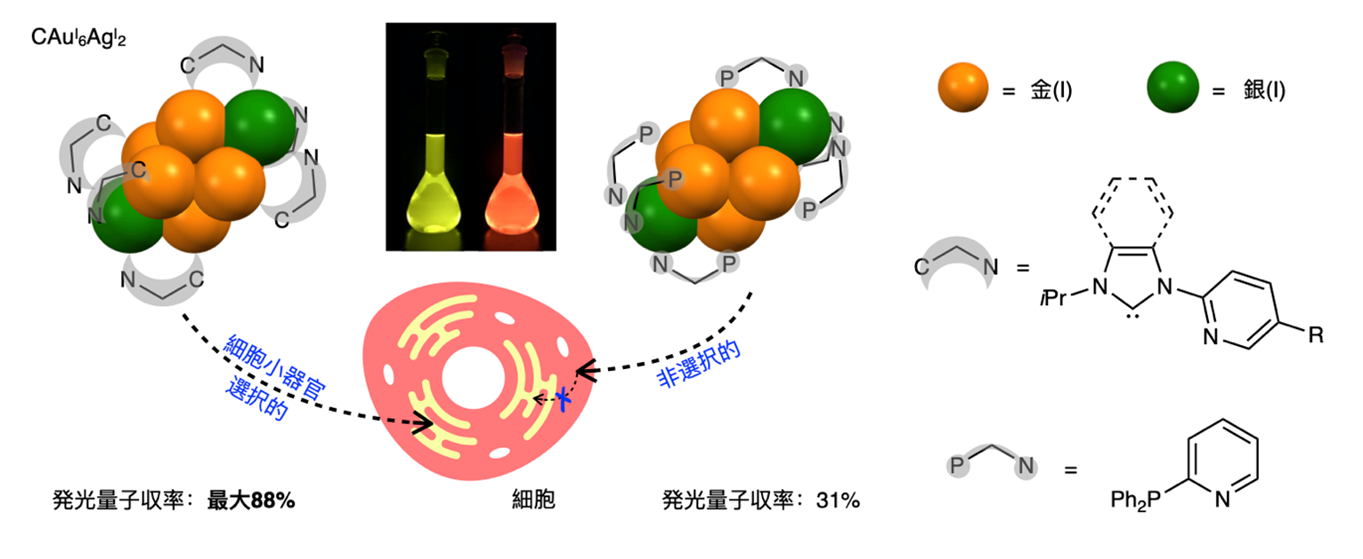
Figure 1: Overview of the research results
Publication details
Sub- to nano-sized metal clusters are important research targets in the field of nanotechnology, and gold nanocluster molecules, which are nano-sized gold atom assemblies, can adopt various molecular and electronic structures. The three-dimensional structure of gold nanoclusters, the electronic structure of gold nanoclusters, the Since the steric structure, electronic state, and reactivity of gold nanoclusters are affected in various ways by the ligands protecting the cluster parts and the metals added to the gold nanoclusters, the precise design of the ligands is very important. In this study, we redesigned and synthesized gold(I) clusters based on a hexanuclear gold(I) cluster with a carbon atom as the central element protected by a nitrogen-containing heterocyclic carbene (NHC) ligand, which was originally developed by the presenter, to improve the photoluminescence (Note 3) of this gold cluster and to apply it to photo-bioanalysis.
To stabilize the cluster structure and control the electronic structure, we designed a carbon-centered gold-silver ( CAuI6AgI2) cluster with a ligand that can bond to two metals. Specifically, a ligand with a 2-pyridyl group on the nitrogen of the NHC ligand was designed, and a carbon-centered gold-silver ( CAuI6AgI2 ) cluster was obtained by adding two equivalents of silver to the gold cluster synthesized in three steps from the ligand precursor. The structure of this cluster molecule was determined by single crystal X-ray structure analysis, and it was confirmed that the same structure remains stable in solution. The gold-silver cluster with benzimidazolidene-type ligands, the most remarkable one, showed yellow photoluminescence with emission maxima around 560 nm in solution as well as in the solid state. The luminescence quantum yield reached up to 88%. Compared to gold and silver clusters with phosphine ligands instead of NHC ligands, the luminescence quantum yield was significantly higher for gold and silver clusters with NHC ligands, although the luminescence lifetimes were comparable. The high luminescence quantum yields of the gold and silver clusters with NHC ligands Theoretical calculations indicate that this higher luminescence quantum yield is due to the enhancement of the radiation process of phosphorescence by the NHC ligand used in the NHC ligand, which shows the usefulness of the NHC ligand.
Thus, gold-silver clusters with NHC ligands showed phosphorescent luminescence with a high quantum yield (Note 4) not only in the solid state but also in solution, which is expected to be used for photo-bioanalysis. Therefore, we investigated the application of this gold-silver cluster to cellular imaging. After examining the conditions for cell introduction, we succeeded in introducing the clusters into various cultured cells, including HeLa cells, HEK293 cells, and COS7 cells, by incubating them in DMSO/PBS solution for 10 minutes. The intracellularly incorporated gold and silver clusters retained their luminescent properties, and their subcellular localization could be visualized under a confocal microscope by excitation with a 405 nm laser light and detection of the 500-550 nm luminescence. Labeling of cellular organelles with commercially available staining reagents revealed that the intracellularly incorporated gold and silver nanoclusters selectively accumulate in the endoplasmic reticulum. In order to analyze the intracellular uptake pathway in detail, we attempted to introduce gold and silver nanoclusters into cells with an inhibitor and found that the intracellular uptake was inhibited by genistein treatment. This indicates that gold-silver nanoclusters with NHC ligands are taken up by the endocytosis pathway. This differs significantly from previously reported nanoclusters with phosphine ligands, which were taken up intracellularly and distributed in the cytosol in a pathway non-selective manner. These experimental results indicate that gold and silver clusters with the newly designed NHC ligands in this study have the potential to control the intracellular uptake pathway and the cell organelles in which they accumulate.
In cell imaging using fluorescent labeling, there is a risk that signals from fluorescent substances that are already present in the cell will be detected as background fluorescence. On the other hand, the newly developed gold-silver nanoclusters measure phosphorescence, which has a longer emission lifetime than fluorescence, and thus are expected to enable imaging that suppresses background fluorescence. Furthermore, observation of cells in which gold-silver nanoclusters were introduced using phosphorescence lifetime microscopy confirmed that the gold-silver nanoclusters incorporated into the cells have a relatively long emission lifetime of 100-200 ns, enabling imaging with suppressed autofluorescence signals.
As described above, in this study, we succeeded in synthesizing gold-silver nanoclusters with NHC ligands and demonstrated the possibility of cell imaging using their phospholuminescence and the possibility of controlling the intracellular uptake pathway and cell organelles in which they are accumulated. The results of this research are expected to open up the chemistry of multi-element metal nanoclusters and provide new material groups and basic technologies for photo-bioanalysis.
Research Team Members:
| Zhen Lei | Project Assistant Professor, Department of Chemistry, Graduate School of Science, The University of Tokyo |
| Mizuki Endo | Assistant Professor, Department of Chemistry, Graduate School of Science, The University of Tokyo |
| Hitoshi Ube | Assistant Professor, Department of Chemistry, Graduate School of Science, The University of Tokyo |
| Takashi Shiraogawa | Doctoral Student (at the time of research), The Graduate University for Advanced Studies |
| Pei Zhao | Project Assistant Professor, Institute for Molecular Science |
| Koichi Osada | Department of Chemistry, Graduate School of Science, The University of Tokyo |
| Xiao-Li Pei | Project Assistant Professor, Department of Chemistry, Graduate School of Science, The University of Tokyo |
| Tomoya Eguchi | (at the time of the research) |
| Toshiaki Kabaike | Professor, School of Bioscience and Biotechnology, Tokyo Institute of Technology |
| Masahiro Ehara | Professor, Research Center for Computational Science and Institute for Molecular Science, National Institutes of Natural Sciences |
| Takemasa Ozawa | Professor, Department of Chemistry, Graduate School of Science, The University of Tokyo |
| Mitsuhiko Shiotani | Professor, Department of Chemistry, Graduate School of Science, The University of Tokyo |
Journal
-
Journal name Nature Communications Title of paper N-Heterocyclic carbene-based C-centered Au(I)-Ag(I) clusters with intense phosphorescence and organelle-selective translocation in cells Authors Zhen Lei, Mizuki Endo, Hitoshi Ube, Takafumi Shiraogawa, Pei Zhao, Koichi Nagata, Xiao-Li Pei, Tomoya Eguchi, Toshiaki Kamachi, Masahiro Ehara*, Takeaki Ozawa*, Mitsuhiko Shionoya1*, Takeaki Ozawa DOI Number
Terminology
1 Nitrogen-containing heterocyclic carbene (NHC)
A two-coordinated six-electron chemical species of carbon stabilized by the introduction of a nitrogen atom and a ring structure. ↑up
Note 2 Phosphorescence
Light produced when a substance returns to its ground state from an excited state by a radiation process in a forbidden transition (a luminescence phenomenon that occurs in an energy transfer process of electrons that is unlikely to occur in a stochastic manner). It has a longer luminescence lifetime than fluorescence luminescence, which is a similar luminescence phenomenon. ↑up
Note 3 Photoluminescence
A phenomenon in which light energy absorbed by a material is emitted as light energy of a different wavelength. ↑up
Note 4 Luminescence quantum yield
The ratio of the number of photons initially absorbed by a material in the process of photoluminescence to the number of photons emitted in a luminescence phenomenon. Normally, 100% is the theoretical maximum value for luminescence phenomena. ↑up


