DATE2025.04.22 #Press Releases
Visualization of the Mechanism by Which a Lasso Peptide Inhibits Receptor Function
ー Potential Applications in the Treatment of Cancers Resistant to Immunotherapyー
Summary
A research group led by Associate Professor Wataru Shibotani (at the time, Assistant Professor at the Graduate School of Science, The University of Tokyo), Professor Osamu Nureki of the School of Science, The University of Tokyo, and Lassogen Inc., in collaboration with the Department of Signal Research at the Keio University School of Medicine (Sakaguchi Laboratory of Biomedical Science), has determined the binding structure of the lasso peptide RES-701 to the endothelin B receptor (ETB receptor), a type of G protein-coupled receptor (GPCR) located on the cell surface, through single-particle cryo-electron microscopy (cryo-EM).
The ETB receptor, a member of the GPCR family, is known to regulate vascular functions and play a role in cancer angiogenesis and immune responses. It has attracted attention as a potential therapeutic target for refractory cancers, and drug development targeting this receptor has been highly anticipated. However, previous efforts have struggled to produce small molecule compounds with sufficient functional activity and selectivity.
RES-701, a lasso peptide, exhibits higher selectivity for the ETB receptor compared to existing drugs and has demonstrated potential as an inverse agonist. Nevertheless, the mechanism by which RES-701 acts on the ETB receptor remained unclear, posing a challenge for its application in drug development.
In this study, the researchers successfully determined the structure of the ETB receptor bound to RES-701—previously a difficult task—by applying a calnexin-fusion method. They visualized how the peptide binds to a specific hydrophobic pocket within the receptor. This binding inhibits the structural changes necessary for interaction between the receptor and G protein, thereby elucidating the mechanism behind the inverse agonist activity.
These findings pave the way for the development of lasso peptide-based therapeutics targeting the ETB receptor and hold promise for applications in the treatment of various diseases, particularly cancers that are resistant to immunotherapy.
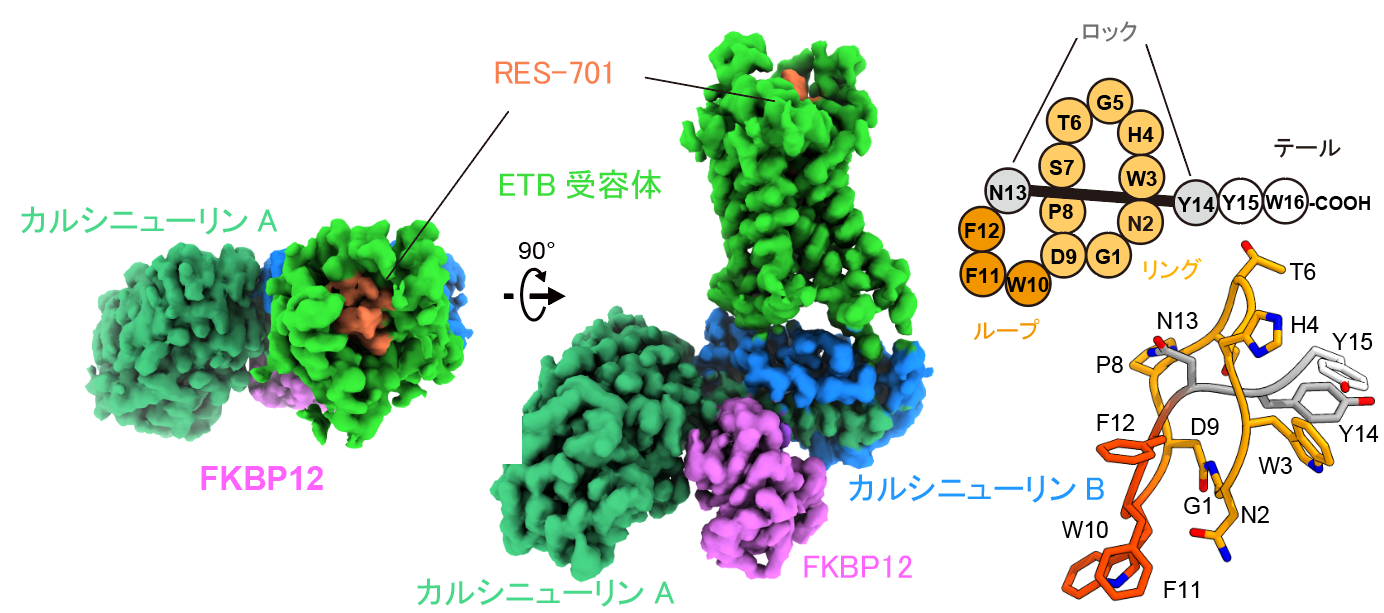
Structure of the ETB Receptor Bound to the Lasso Peptide RES-701
Links
Keio University school of medicine
Journals
-
Journal name Nature CommunicationsTitle of paper


