DATE2025.03.06 #Press Releases
Identification of Grr1, a degradative factor that promotes the endoplasmic reticulum stress response.
--Mechanism of Translation Regulation Caused by Ribosomal Ubiquitination--
Summary
A research group led by Professor Toshifumi Inada from the Laboratory of RNA Control at the Institute of Medical Science, The University of Tokyo, and the Department of Biological Sciences, Graduate School of Science, The University of Tokyo, along with graduate student Nichika Sato from the same department, has elucidated the mechanism of translation regulation caused by ribosome ubiquitination under endoplasmic reticulum (ER) stress conditions.
In this study, screening using a library of E3 ubiquitin ligase-deficient budding yeast strains identified Grr1 as a novel factor involved in the unfolded protein response (UPR). Further detailed analysis revealed a molecular mechanism in which "the degradation factor Grr1 degrades a deubiquitinating enzyme, thereby indirectly increasing the level of ribosome ubiquitination and promoting the translation of HAC1 mRNA, a transcription factor."
Compared to previous studies, this research provides a novel perspective on ER stress response by analyzing translation regulation driven by ribosome ubiquitination. These findings are expected to contribute to the development of new therapeutic strategies and drug discovery in the future.
The research results were published online in Nature Communications on March 4, 2025.
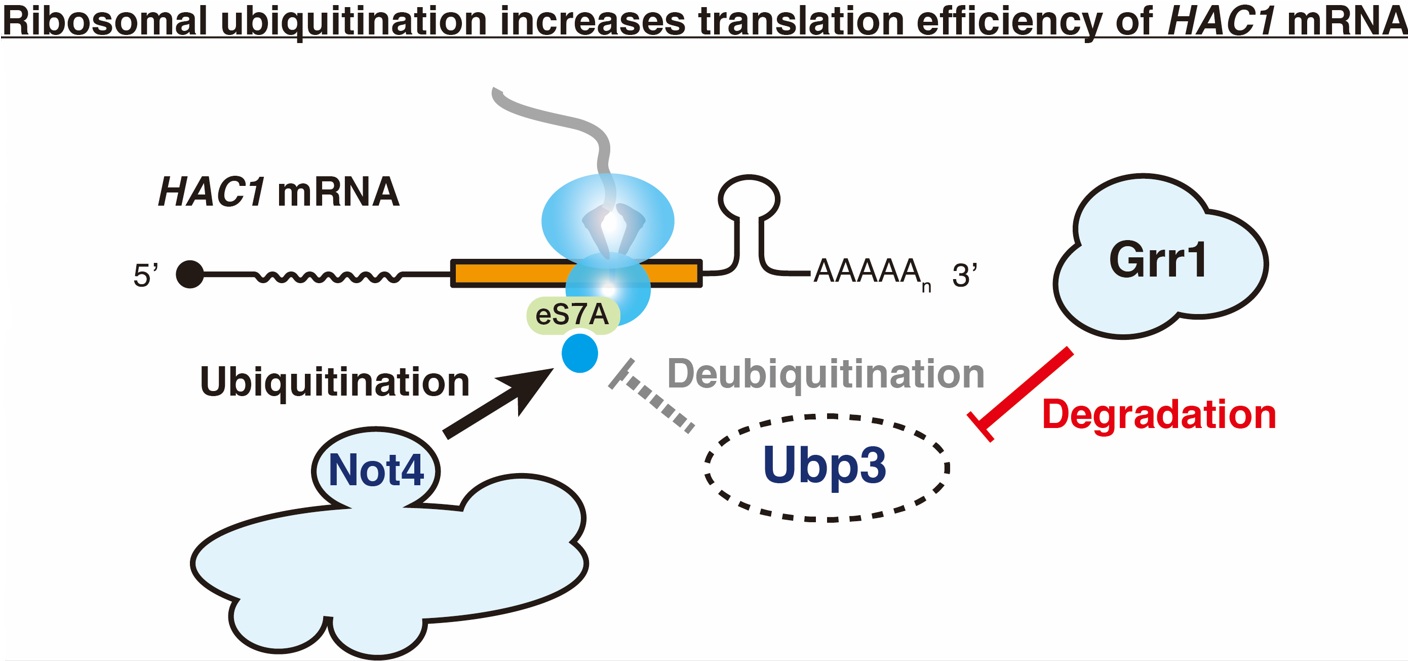
Ribosomal ubiquitination-induced translational regulation under endoplasmic reticulum stress conditions
Related Link
The University of Tokyo Institute of Medical Science
Published Journals
-
Journal name Nature CommunicationsTitle of paper


