DATE2025.03.11 #Press Releases
Discovered a novel peptide sequence that stops protein synthesis.
Summary
Proteins in the organism are synthesized by the cellular machinery called ribosomes based on genetic sequences encoded in DNA, a process known as "translation." It is commonly assumed that ribosomes can synthesize any protein; however, it has become clear that certain sequences, known as "ribosome arrest sequences," pose challenges to synthesis. Various amino acid sequences have been identified as difficult to translate, and some of them have been reported to play roles in gene expression regulation. So far, the extent of such sequences and the underlying principles governing their difficulty in translation remain incompletely understood.
A research team led by Associate Professor Yuhei Chadani of Okayama University, along with Akinao Kobo (a doctoral student), Assistant Professor Tatsuya Niwa, and Professor Hideki Taguchi of Tokyo University of Science, as well as Yushin Ando (a master's student), Associate Professor Yuzuru Itoh, and Professor Osamu Nureki of the University of Tokyo, developed a novel technology to identify previously unknown ribosome arrest sequences using Escherichia coli as a model organism. Through large-scale analysis using this technique, they identified two new ribosome arrest sequences, pepNL and nanCL, from E. coli genes. Furthermore, structural analysis of ribosomes stalled by PepNL using cryo-electron microscopy revealed that the PepNL peptide forms an abnormal hairpin-like conformation within the ribosomal tunnel, leading to translation arrest.
This study was published online in the British academic journal Nature Communications on 8 March 2025.
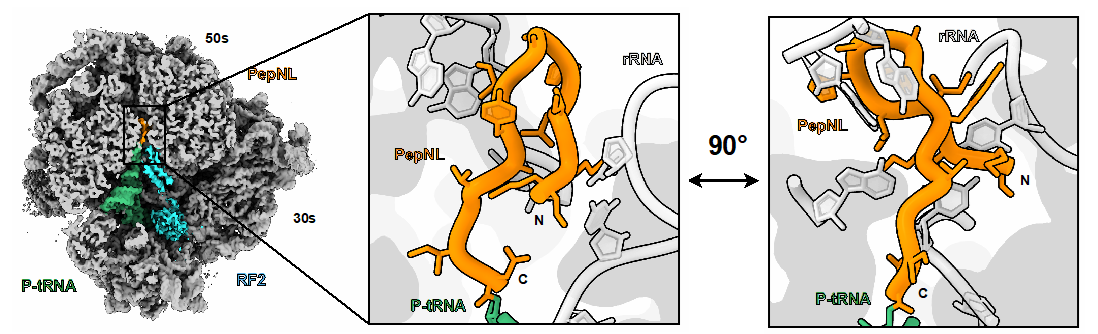
Figure: E. coli PepNL nascent peptide forming a mini-hairpin structure in the ribosomal tunnel
(cryo-EM structural analysis)
Related link: Okayama University, Institute of Science Tokyo
Journal
-
Journal name Nature CommunicationsTitle of paper A mini-hairpin shaped nascent peptide blocks translation termination by a distinct mechanism


