DATE2025.02.06 #Press Releases
How does cesium adsorb into the soil?
-A nanoscale world revealed by high-precision simulations and experiments-
Summary
In this study, the researchers succeeded in capturing the adsorption reaction on a nanoscale, showing how caesium (Cs) is adsorbed on clay minerals. Furthermore, it was clarified that Cs is strongly adsorbed due to the atomic structure of the surrounding clay minerals, despite the relatively weak ionic bonding of Cs in the adsorption state.
For the geological disposal of radioactive waste and decontamination of radioactive materials, the behaviour of radioactive elements in the soil as metal ions needs to be understood. In particular, Cs, an important element in these issues, is considered to be easily adsorbed on clay minerals in the soil. However, the adsorption reactions of Cs on clay minerals are complex and remain largely unresolved.
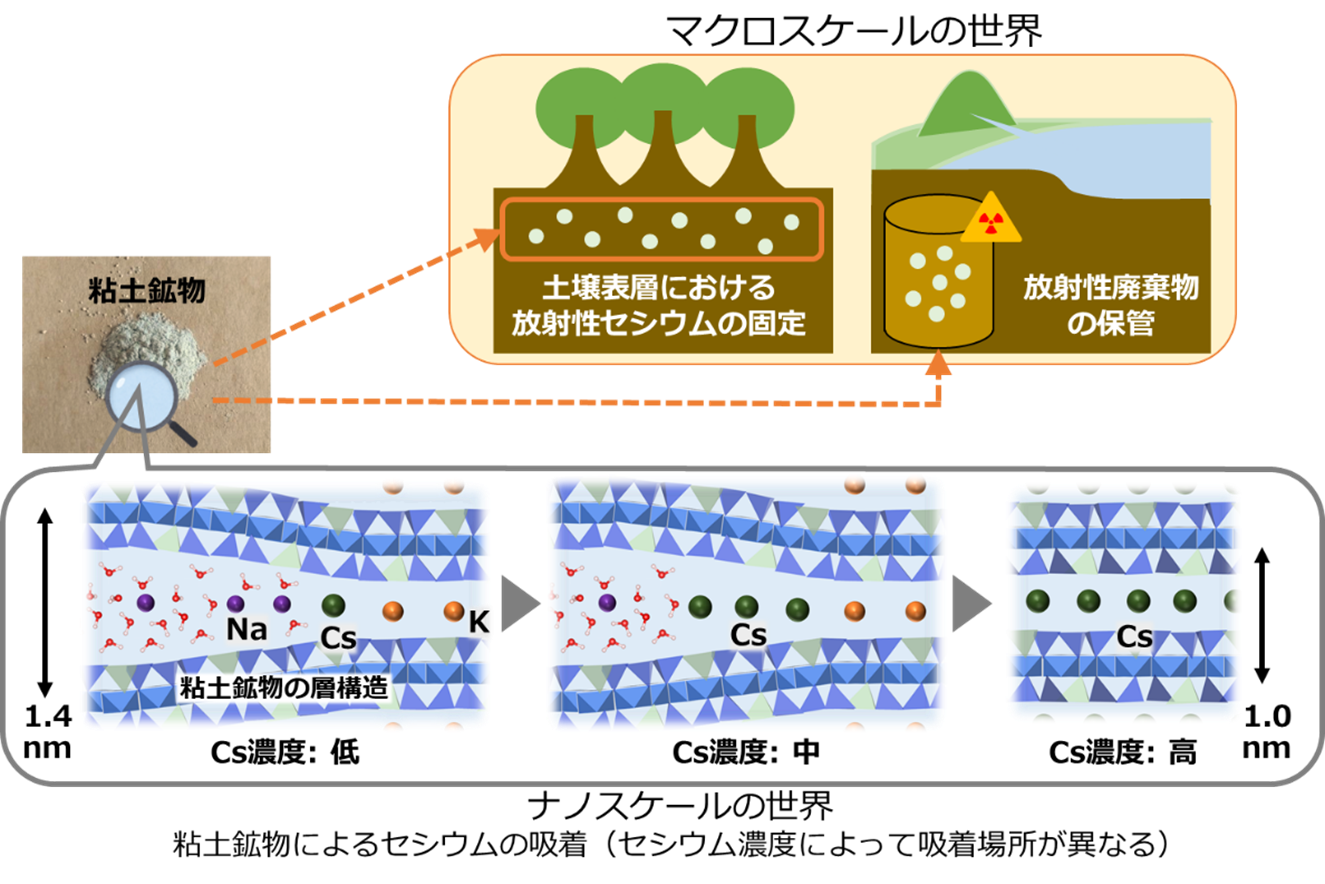
Clay minerals have complex structures at the nanoscale, so to understand adsorption reactions on clay minerals, it is necessary to clarify what is happening at the nanoscale and what the structure is like. For example, clay minerals have a structure like a sandwich, with small-grained sodium (Na) and other ingredients sandwiched between bread, but it has been unclear how the larger-grained Cs enters the sandwich when it replaces the Na and other ingredients. This is because experiments to observe nanoscale information have so far been carried out at concentrations several orders of magnitude higher than the actual environmental Cs concentration, and only the final state, in which only Cs is already sandwiched in the bread, could be observed.
In this study, therefore, Extended X-ray Absorption Fine Structure (EXAFS) measurements were carried out on a wide range of samples, which provide nanoscale information even at relatively low concentrations. The results revealed a systematic change in the distance between Cs and its neighbouring atoms as the Cs concentration increases. In addition, supercomputer simulations were carried out for several possible models. By comparing these with the experimental results, it was possible to estimate how the ‘contracted interlayer’, the sandwich structure sandwiching Cs, spreads with Cs concentration as Cs is first adsorbed at the adsorption site ‘frayed edge’, where the sandwich structure opens at the edge, and gradually the ‘frayed edge’ transitions to the neighbouring site. This could be estimated.
Furthermore, this study showed that the High-Energy Resolution Fluorescence Detection(HERFD)-X-ray Absorption Near Edge Structure (XANES) method can be used to assess the bonding properties of the target atoms, and that Cs adsorbed on clay minerals forms ionic bonds regardless of the adsorption site.
This study has allowed a detailed clarification of the adsorption reaction of Cs on clay minerals. This knowledge is expected to lead to the solution of socially important issues such as the prediction of the environmental behavior of Cs and the study of more accurate safety assessment and treatment methods in the treatment of radioactive waste, where the strong adsorption capacity of Cs on clay minerals is important.
This research was conducted in collaboration with the Japan Atomic Energy Agency (President: Masanori Oguchi, hereafter referred to as ‘JAEA’). Akiko Yamaguchi and Masahiko Okumura, AI/DX Research and Development Office, Center for Computational Science and e-Systems, JAEA; Naomi Kawamura, Senior Researcher, Japan Synchrotron Radiation Research Institute; and Professor Yoshio Takahashi, Director, Isotope Research Centre, School of Science, The University of Tokyo, among others. The research results were published in Science of The Total Environment, Elsevier, USA, on 24 January (local time).
links: Japan Atomic Energy Agency (JAEA)、 Isotope Science Center, The University of Tokyo
Journals
-
Journal name Science of The Total EnvironmentTitle of paper


