DATE2024.11.07 #Press Releases
Elucidating the mechanism of drug selection for A3 receptors involved in tumor, inflammation and neural activity.
Summary
A research group led by Professor Osamu Nureki at the School of Science, The University of Tokyo, and Professor Fan-Yan Wei at the Institute of Development, Aging and Cancer, Tohoku University, has characterized the three-dimensional structure of the signaling complex between the adenosine A3 receptor and the trimeric G protein Gi activated by adenosine or its modifications by cryo-EM (cryo-EEM)-EM) and single particle analysis.
A3 receptors are abundant in cancer cells and immune cells and are attracting attention as new drug targets. In particular, a modified adenosine, m6A, was known to selectively activate A3 receptors among adenosine receptors, but the detailed mechanism was not known. In this study, we determined the complex structure of the A3 receptor bound to m6A and the trimeric G protein Gi, and newly discovered that the modified adenosine i6A selectively activates the A3 receptor. They also found that the A3 receptor has a hydrophobic pocket that is not found in other receptors, and that selective drugs exert their effects by binding to this pocket.
These results are expected to lead to the development of new drugs targeting the A3 receptor and contribute to the treatment of cancer and inflammation.
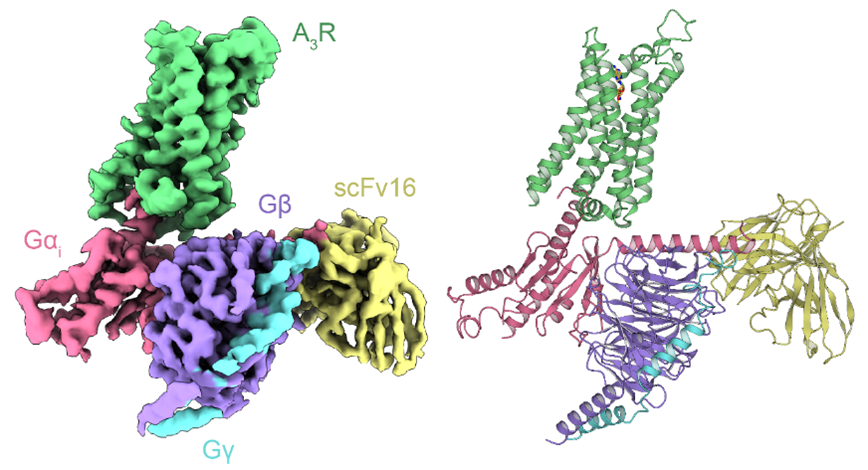
Overall structure of A3R-Gi complex
Links:Tohoku University, Institute of Development, Aging and Cancer, Tohoku University. (in Japanese)
Journals
-
Journal Nature Communications Title of paper


