DATE2022.09.20 #Press Releases
Elucidation of the active-type structure of the LPA receptor involved in cancer and pneumonia
Disclaimer: machine translated by DeepL which may contain errors.
-Contribute to drug discovery research by clarifying interactions with new compounds
Hiroaki Akasaka (Master's Program, Department of Biological Sciences)
Wataru Shihoya (Assistant Professor, Department of Biological Sciences)
Osamu Nureki, Professor, Department of Biological Sciences
Key points of the presentation
- We have succeeded in structural analysis of the complex of LPA receptor ( LPA1) and Gi protein trimer bound by a novel agonist.
- The binding mode and activation mechanism of the agonist were clarified, and the difference between LPA and the agonist working in vivo was shown. We also observed structural polymorphism at the receptor-protein interface and proposed that it may represent the dissociation process of the receptor.
- This study paves the way for the design of more effective and safer agonists targeting LPA1.
Publication Summary
Lysophosphatidic acid (LPA) (Note 1) is one of the lipid molecules that function as intercellular signaling molecules in vivo. 6 types of LPA receptors are known to accept LPA, and all LPA receptors are membrane proteins of the type called G-protein coupled receptors (GPCR) (Note 2) (Note 2). Among them, LPA1 is known to be associated with fibrosis of lung tissue and is attracting attention as a therapeutic target for various diseases including cancer. However, the structure of LPA1 reported so far is only the inactive form bound to an antagonist, and the detailed activation mechanism by an agonist was unknown.
In this study, a research group led by Graduate Student Hiroaki Akasaka, Assistant Professor Wataru Shihoya, and Professor Osamu Nureki at the Graduate School of Science, The University of Tokyo, used a novel agonist that activates LPA1 and determined the steric structure of the activated LPA1 complex with the Gi protein trimer (Note3 ) using cryo-EM ( ). Based on this structure, we determined how the agonist transforms the receptor into the active conformation and the mechanism of activation. We also observed structural polymorphism at the LPA1-G protein interface and showed that this structural polymorphism may represent the dissociation process of the G protein. The results of this study are expected to contribute to drug discovery research by enabling the design of more effective and safer agonists.
The research results were published in the British scientific journal Nature communications on September 15, 2022 (Japan time).
Publication details
Background of the research
G protein-coupled receptors (GPCRs) exist on the membrane and change to their active conformation upon binding to agonists (agonists). Among GPCRs, six types of LPA receptors have been identified that specifically accept a molecule called lysophosphatidic acid (LPA). One of them, LPA1, is known to be associated with migration and differentiation of neurons and fibrosis of tissues, and its antagonists are attracting attention as therapeutic drug targets for various diseases including cancer. There are also reports that agonists have inhibitory effects on obesity and urine leakage, and development of such agonists is also underway. since LPA is easily degraded in vivo, development of potent and metabolically stable agonists that can replace LPA is expected in the search for clinical applications and therapeutic effects of LPA receptors. However, the structure of LPA1 reported so far is only the inactive form bound to an antagonist, and the detailed mechanism of activation by an agonist has not been clarified.
Description and Results of the Study
In this study, the crystal structure of LPA1 activated by a novel agonist in complex with a Gi protein trimer was determined by cryo-EM (Fig. 1). The agonist used in this study is similar to LPA, but has a lower 50% effective concentration ( EC50 ) for LPA1, about 1/30, and activates LPA1 more potently.
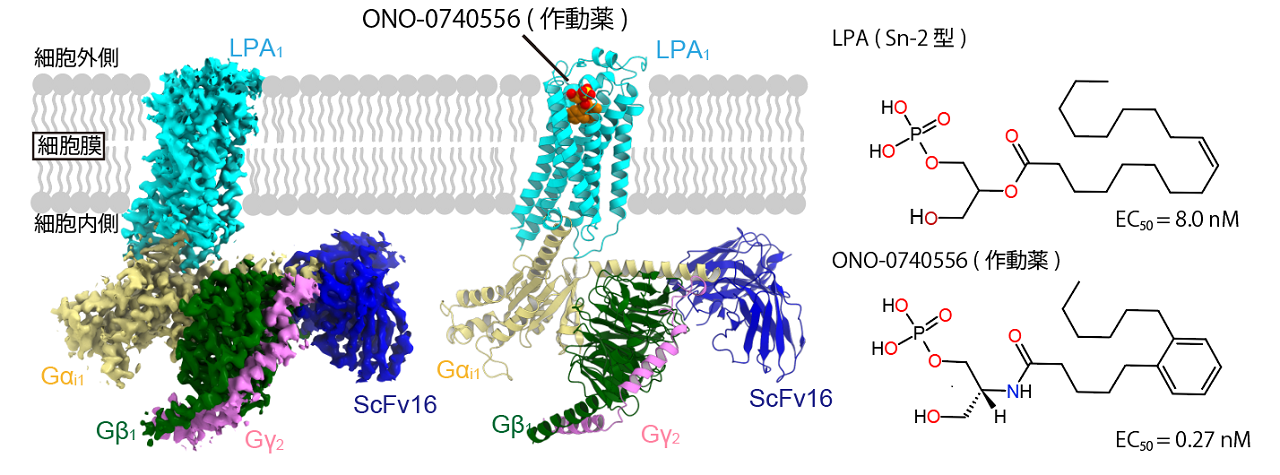
Figure 1: Chemical formulas of LPA and agonist, and structure of LPA1 complex
Comparison with the structure of the inactive form reveals that binding of the agonist causes movement of the seventh transmembrane helix (TM7) and that it acts on residues at the bottom of the binding region to change its arrangement. These movements act as a switch, transforming the entire receptor into the active conformation and allowing binding to the G protein (Figure 2).

Figure 2: Activation of LPA1 by agonists
Figure superimposed on the structure of the inactive form (Chrencik et al., 2015). Binding of the agonist changes the structure of the binding region (left, center). LPA1, transformed into the active conformation, has a large outward movement of transmembrane helix 6 (TM6), allowing binding to G proteins (right).
Comparison with a structural model of LPA-bound LPA1 published during the submission of this paper revealed why the agonist has a higher affinity compared to LPA: while the recognition mechanism of the phosphate group head was almost identical between LPA and the new agonist, the position of the hydrocarbon chain inside the binding region was different The position of the hydrocarbon chain fold inside the binding region is different between LPA and the new agonist. This results in a change in the interaction with hydrophobic residues, causing differences in affinity for LPA1 (Figure 3). Such information will provide useful clues for the design of future compounds targeting LPA1 and is expected to contribute to drug discovery research.

Figure 3: Comparison with LPA-bound structure
Figure superimposed on the LPA-bound structure (S. Liu et al., 2022). The binding region is seen from the outside of the cell (left) and from the side (right); LPA is bent around TM5, whereas ONO-0740556 is bent around TM7.
In this study, we also determined structures with different binding states at the LPA1-G protein interface. Some of the structures were found to be distant from LPA1 and Gi, indicating that this difference in binding state may represent the dissociation process of the G protein (Fig. 4). This is expected to advance future studies of GPCR-G protein complexes.

Figure 4: Dissociation process of Gi
Diagram superimposing the most stable structure and structural polymorphisms (states 1 and 2). From state 1 to 2, Gi is moving away from LPA1.
This study was supported by Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS), Grant-in-Aid for Scientific Research (S) "Structural Dynamics of GPCRs in Biological Environment" (Project Leader: Osamu Nureki, 21H05037), Grant-in-Aid for Scientific Research (B) "GPCR Drug Discovery Research Using Cryo-EM" (Project Leader: Wataru Shihodani, 22H02751), and the Ono Foundation for Medical Research. ) and "Structural analysis of lysophosphatidic acid receptor LPA1 and development of NAM" (PI: Wataru Shihoya) funded by the Ono Medical Research Foundation. This research was also conducted as part of the AMED "Platform for Supporting Life Science Research including Drug Discovery" and "Incubate Type of Support Program for Innovative R&D on Advanced Technology," which aims to link the results of outstanding life science research to the practical application of pharmaceuticals and other products by opening large facilities such as synchrotron radiation facilities to external users. The project was supported by the Platform for Advanced Technology Support for Drug Discovery, etc. (BINDS).
Journals
-
Journal name Nature communications Title of paper Structure of the active Gi-coupled human Lysophosphatidic Acid Receptor 1 complexed with a potent agonist Author(s) Hiroaki Akasaka, Tatsuki Tanaka, Fumiya K. Sano, Yuma Matsuzaki, Wataru Shihoya*, Osamu Nureki*, and Yuma Matsuzaki DOI Number
Terminology
1 Lysophosphatidic acid (LPA )
Lysophosphatidic acid (LPA) is a lipid molecule that functions as a signaling molecule between cells in vivo. Six types of LPA receptors ( LPA1-LPA6 ) have been identified, and LPA molecules trigger various cellular responses such as cell proliferation and cell migration through these receptors. LPA molecules trigger various cellular responses, such as cell proliferation and cell migration, through these receptors. ↑up
Note 2 G protein-coupled receptor (GPCR)
GPCRs are membrane proteins expressed on the plasma membrane and consist of seven α-helices that penetrate the membrane. They form the largest family of membrane receptor proteins and are activated by the binding of specific ligands in the extracellular region and transduce signals by activating intracellular G protein trimers. Because of their important role in regulating diverse physiological functions in the body, more than 30% of approved drugs target GPCRs. ↑up
Note 3 Cryo-electron microscopy
This device is used to observe samples by irradiating electron beams against molecules such as proteins under liquid nitrogen (-196°C) cooling. It is used in the single-particle analysis method, which determines the three-dimensional structure of proteins, nucleic acids, and other biological macromolecules by taking numerous images of them. ↑up
Note 4 G protein, Gi protein trimer
G proteins are GTP-binding proteins involved in intracellular signal transduction and are composed of a trimer of Gα, Gβ, and Gγ subunits. The G protein trimer bound to an activated G protein-coupled receptor (GPCR) undergoes a GDP-GTP exchange reaction and dissociates into two subunits, Gα and Gβ-Gγ. The dissociated subunits bind and activate downstream signaling factors, resulting in a variety of cellular signaling responses, including Gs , Gi ,Gq/11, andG12/13 subunits. activity, thereby transducing an inhibitory signal. ↑up


