DATE2024.03.29 #Press Releases
Cryo-EM reveals the full-length structure of the cargo receptor, which is essential for normal secretion of blood coagulation factors
Disclaimer: machine translated by DeepL which may contain errors.
--The Full-Length Structure of the Tetramer and the Regulation Mechanism by Zinc Revealed, Defying Conventional Theories--
Tohoku University
Kyushu University
School of Science, The University of Tokyo
High Energy Accelerator Research Organization
Summary of Presentations
Secreted proteins such as blood coagulation factors are synthesized in the endoplasmic reticulum (ER) of cells and then recognized by specific cargo receptors as cargoes and efficiently secreted out of the cells. Abnormalities in cargo transport are known to cause various genetic disorders such as hematologic diseases. ERGIC-53 and its cofactor MCFD2 were identified as cargo receptors that play a central role in cargo transport about 40 years ago, and structure-function studies have been conducted mainly on the region (domain) of ERGIC-53 that recognizes sugar chains. However, its full-length structure has not yet been determined, and the mechanism by which it recognizes and transports various carbohydrates in its full length has not yet been elucidated.
A research group led by Assistant Professor Satoshi Watanabe and Professor Kenji Inaba of the Institute of Multidisciplinary Research for Advanced Materials (IMRAM), Tohoku University, has determined the steric structure of the complex of full-length ERGIC-53 and the auxiliary factor MCFD2 for the first time in the world using cryo-EM single particle analysis. The structural analysis revealed that the full-length structure consists of a head region, a stalk region, and a transmembrane region, and that the overall structure resembles a four-leaf clover. We also succeeded in visualizing the dynamic conformational changes of the full-length structure of ERGIC-53, revealing a cargo recognition mechanism that utilizes flexible conformational changes. In addition, we succeeded in determining the high-resolution structure of the head region of the ERGIC-53-MCFD2 complex, revealing not only the detailed molecular basis of tetramer formation, but also a new zinc-binding site in MCFD2, suggesting a zinc-enhanced mechanism for cargo dissociation downstream of the secretory pathway. This finding suggests a mechanism by which the dissociation of cargo downstream of the secretory pathway is promoted by zinc.
The research results were published in Nature Communications on March 16, 2024.
This research result was obtained in collaboration with Project Associate Professor Yoshiaki Kise and Professor Osamu Nureki at the School of Science, The University of Tokyo, and with Project Researcher Kento Yonezawa (currently at Nara Institute of Science and Technology) and Professor Nobutaka Shimizu at the Institute for Materials Structure Science, High Energy Accelerator Research Organization (KEK), Japan.
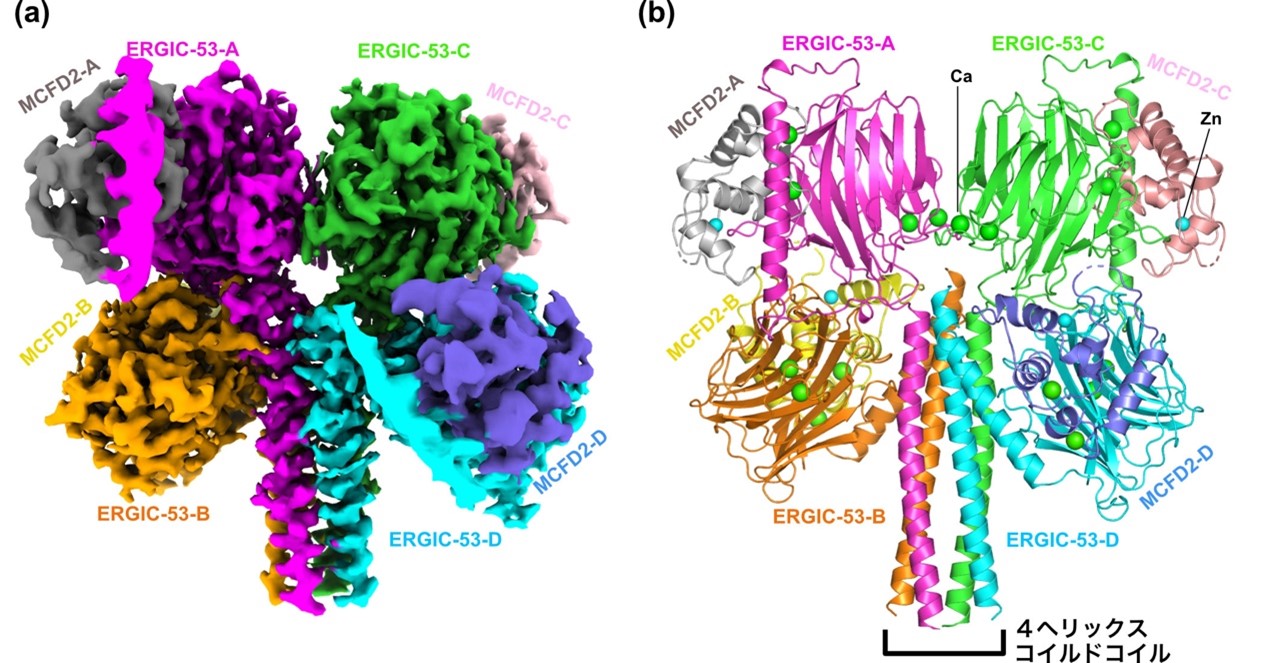
Figure: High-resolution structure of the head region of the ERGIC-53-MCFD2 complex.
(a) Density map obtained by cryo-EM. (b) Molecular structure model of the constructed head region, in which four long α-helices in the stalk domain of ERGIC-53 come together to form a 4-helix-coiled coil, forming a tetramer.
For more information, please visit the Tohoku University website (in Japanese).
Journals
-
Journal name Nature CommunicationsTitle of paper


