Disclaimer: machine translated by DeepL which may contain errors.
Microscopic Observations of Receptor Signaling
Wataru Shihoya (Assistant Professor, Department of Biological Sciences) |
Osamu Nureki, Professor, Department of Biological Sciences |


Adrenaline stimulates the sympathetic nervous system by activating β-receptors, which increases heart rate and blood pressure. The β-receptor is a G-protein coupled receptor and undergoes a conformational change when it receives adrenaline in its extracellular pocket. As a result, it is able to activate intracellular G proteins, thereby enabling signal transduction across the plasma membrane.
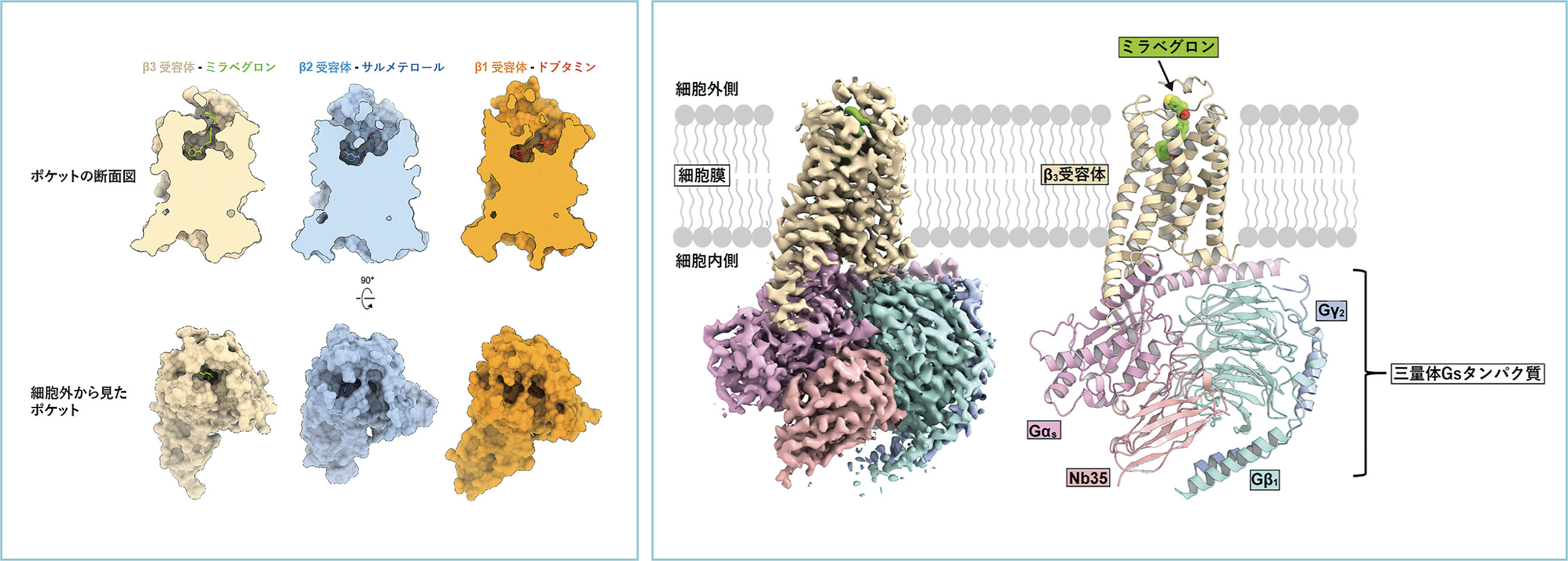
There are three types of β-receptors (β1-3), and drugs targeting β1 and β2 are typical drugs for heart disease and asthma. On the other hand, β3 receptors are abundantly expressed in adipocytes and are responsible for heat production and lipolysis. Polymorphisms of the β3 receptor gene are well-known as thrifty genes because they lower basal metabolism and make people more likely to gain weight. β3 receptors are involved in smooth muscle relaxation of the bladder, and mirabegron (product name: Benidus), a selective stimulator of β3 receptors, is a treatment for overactive bladder. Since β1-3 receptors have different physiological effects, the selectivity of the drug for each receptor is important to reduce side effects. Studies on β1 and β2 receptors have been progressing, and their atomic-level structures have been elucidated one after another, thus the mechanism of action of drugs has been understood. However, the steric structure of the β3 receptor has not yet been elucidated, and our understanding of the selectivity of β-receptor stimulants has been limited.
Therefore, we determined the steric structure of mirabegron to elucidate why it can selectively activate β3 receptors. We produced a large amount of β3 receptor protein and purified it to a high purity with mirabegron bound to it. We then mixed and separated the trimeric Gs proteins to form a signal transduction complex that activates the G protein. A grid of the complex trapped in thin ice was prepared and photographed by cryo-electron microscopy. The structure of the signaling complex was successfully determined by a technique called single-particle analysis, in which 400,000 particles were extracted from the captured image (Figure (right)).
The drug-binding pocket of the β3 receptor extends perpendicular to the plasma membrane, and the elongated form of mirabegron fits well into the pocket (Figure left). The entrance of the drug-binding pocket of β3 receptors is narrower than that of β1 and β2 receptors, and the amino acid residues constituting the entrance are less conserved among β receptors. Mirabegron can bind with high affinity by fitting into the narrow drug-binding pocket of β3 receptors and does not bind well to β1 and β2 receptors with an open entrance. Conversely, β1- and β2-selective stimulants cause steric hindrance to the narrow β3 receptor drug-binding pocket. This difference in pocket shape was found to be important for the β3 receptor selectivity of mirabegron. Now that the steric structures of all β-receptors have been clarified, it is expected that β-receptor-targeted drugs with enhanced selectivity for each receptor and fewer side effects will be created.
The research results were published in Molecular Cell 81, 3205 (2021) by C. Nagiri et al.
(Press release on July 27, 2021)
Published in Faculty of Science News, November 2021
The Forefront of Research Communicated to Faculty Students>
<