Disclaimer: machine translated by DeepL which may contain errors.
The Beginning of Crystals Revealed by Electron Microscopy
Eiichi Nakamura ( Department of Chemistry, University Professor / Emeritus Professor) |
Takayuki Nakamuro, Project Assistant Professor , Department of Chemistry |


In order to get a glimpse of molecular behavior, we need to be creative. Carbon nanotubes (CNTs) are an appropriate material for molecular observation because molecules can be placed inside or attached to the outside. Taking advantage of this property, in 2007 we first captured the dynamic behavior of organic molecules by atomic-resolution transmission electron microscopy (TEM). This methodology, which we named SMART-EM, combines single-molecule, real-time, and atomic resolution, and can record slow-motion images of phenomena occurring in the molecular world. In this study, using sodium chloride (NaCl), which is familiar to us in our daily life, we succeeded in recording the birth of a crystal nucleus inside a conical CNT.
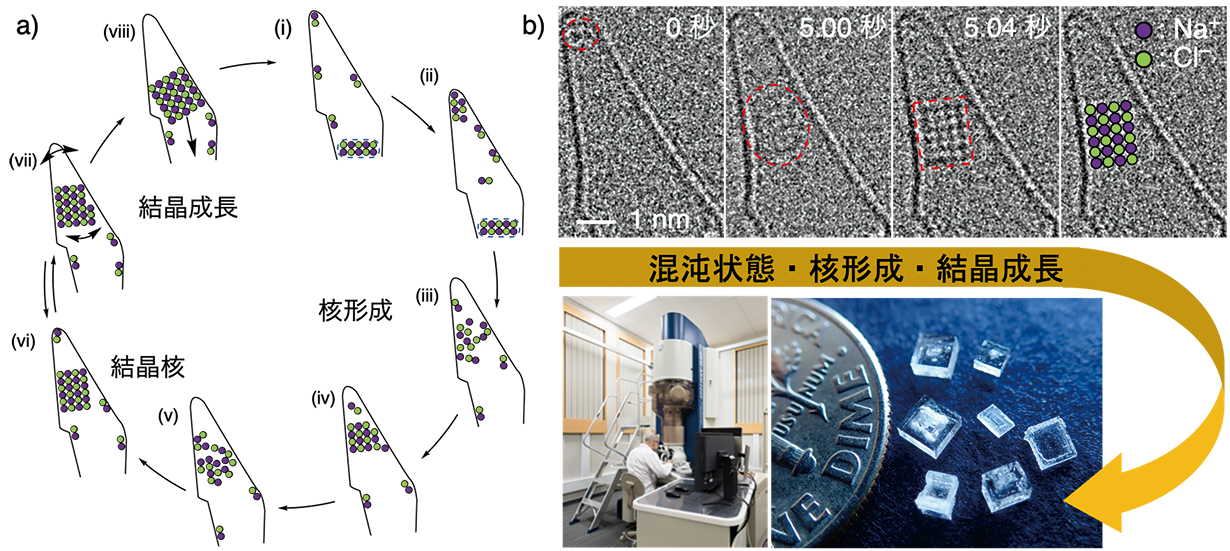
Figure 1a is an overview of the slow-motion video. Molecules were assembled at the tip of the tiny test tube (i-iv), crystal nuclei were born (v-vi), and the crystal nuclei grew as crystals (vi-viii). 9 times the crystallization process was repeated in the same container within 152 seconds, providing a key to understanding the full extent of the phenomenon. Figure 1b shows the actual TEM image and the overall picture of the study. The structure on the line is the wall of the conical CNT, and the area enclosed by the red dotted line corresponds to the NaCl aggregate. The irregular structure of the NaCl aggregate (5.00 sec) was converted into a NaCl crystal nucleus with a periodic structure of 4 atoms wide and 6 atoms high (5.04 sec). The time between the two events was 0.04 sec, which is the moment when the crystal nucleus was born (corresponding to Fig. 1a, v-vi). In fact, the crystal nucleus we observed has a series of atoms in the depth direction, and appears to be a rectangular body consisting of a total of 96 atoms. It is interesting to note that NaCl nuclei of about 1 nanometer (one billionth of a meter) in size appeared nine times at the tip of the CNTs with the same size and repeatability. The crystal nucleus that emerged from the formation process gradually increased in size (vi-viii) and eventually grew into a shiny crystal of the size we see today.
Crystallization is a common phenomenon that is also treated in science experiments in elementary education. In 2021, we succeeded in obtaining slow-motion images of the crystallization phenomenon using the SMART-EM method, an analytical technique that traces changes in the structure and shape of individual molecules with atomic resolution using an atomic-resolution electron microscope. This method has revealed the details of the phenomenon at the molecular level. The present study must have been accepted with surprise, since NaCl crystals have a square shape from the moment of their birth. Further development of in situ studies of atoms and molecules will reveal new science beyond our imagination in the future.
 |
||
| Figure: NaCl crystallization study. a) Schematic of the observation results. b) TEM can reveal the whole picture of crystallization. A photograph of a square NaCl crystal is shown at the lower right. | ||
The results of this study were published in T. Nakamuro et al. 143, 1763 (2021), and was selected for JACS Spotlights as a particularly important result.
(Press release, January 22, 2021)
Published in the May 2021 issue of Faculty of Science News
Communicating to Faculty Research Students >


