Disclaimer: machine translated by DeepL which may contain errors.
Tetrahedral "Chiral Zinc" Complexes with Remarkable Stability
Hitoshi Ube( Assistant Professor, Department of Chemistry) |
Mitsuhiko Shiotani, Professor , Department of Chemistry |


Chirality is the property that a compound does not overlap with its mirror image, and a pair of stereoisomers in a mirror-image relationship are enantiomers. The enantiomers have different responses to electromagnetic waves and different physiological activities based on intermolecular interactions, so the separation of enantiomers is an important issue in chemistry. The conventional method of creating chiral metal complexes is to bond chiral species to a metal. On the other hand, "asymmetric metal" complexes, in which all the different achiral (non-chiral) bonding species (ligands) are attached to the metal, allow the metal as the chiral center to activate the substrate in the catalytic reaction. Therefore, asymmetric metal complexes have attracted much attention in recent years as a way to broaden the scope of design of asymmetric metal catalysts. However, conventional "asymmetric metal" complexes are mainly of stable octahedral type, and tetrahedral "asymmetric metal" complexes are difficult to obtain optically pure complexes because of the rapid racemization of the more abundant enantiomer to the other enantiomer. This study was initiated four years ago with the aim of establishing a method for the synthesis of tetrahedral "asymmetric metal" complexes that have a chiral center only on the metal and can remain stable in an optically pure state, and furthermore, to apply this method to asymmetric catalysis.
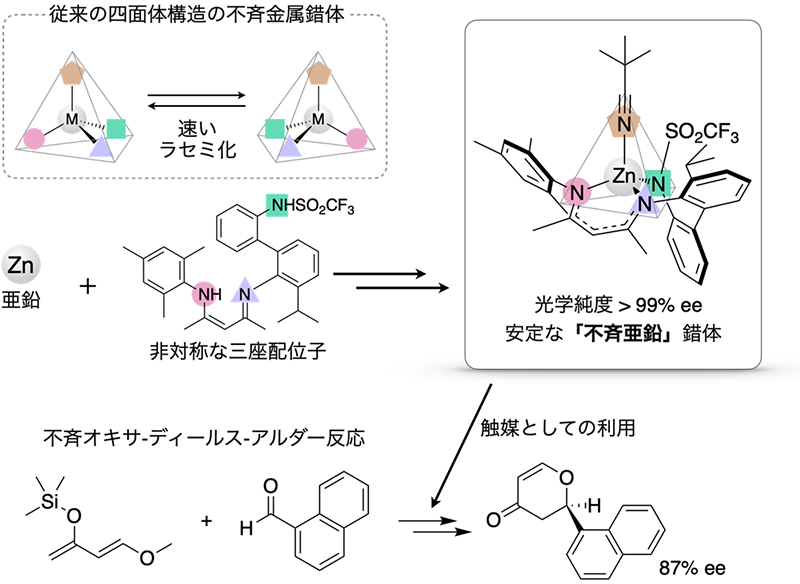
The approach to the synthesis of optically pure tetrahedral "asymmetric metal" complexes started with the design and synthesis of asymmetric tridentate ligands, taking into account the strong zinc-ligand bond and rigid structure. This design prevented racemization due to steric inversion on the zinc chiral center, and the remaining coordination site could be used for asymmetric catalysis.
First, the tridentate ligand was reacted with a zinc ion, and then the chiral ligand was attached to the zinc, resulting in asymmetric induction with one of the two enantiomers as the main product. Further, by replacing the chiral ligand with an achiral ligand, an "asymmetric zinc" complex with a chiral center only on zinc and an optical purity of almost 100% was isolated as a single crystal. This optical purity was maintained even after heating in benzene at 70°C for 24 hours, indicating that the racemization of the complex was remarkably slow. Furthermore, the zinc complex was successfully applied to the asymmetric oxa-Diels-Alder reaction (reaction yield: 98%, asymmetric yield: 87%ee (mirror excess)). This result is attributed to the remarkable conformational stability of the zinc chiral center.
 |
||
| Design concept of "asymmetric zinc" complexes and their application to asymmetric catalysis. | ||
As described above, in this study, we have achieved for the first time in the world the synthesis of tetrahedral "asymmetric zinc" complexes that are stable in an optically pure state and their application to asymmetric catalytic reactions. This research is expected to open up the chemistry of "asymmetric metal" complexes, in which the metal is the only chiral center, and to provide new materials and technologies for the asymmetric synthesis of pharmaceuticals and optical materials.
This work was supported by K. Endo et al. Nature Communications 11, 6263 (2020). Published in.
(Press release, December 9, 2020)
Published in the March 2021 issue of Faculty of Science News
Communicating to Faculty Research Students >


