DATE2021.11.19 #Press Releases
Elucidation of the mechanism of polyamine transport into the cell
Disclaimer: machine translated by DeepL which may contain errors.
Atsuhiro Tomita (Doctoral Student, Department of Biological Sciences)
Takashi Oho (Associate Professor, Asahikawa Medical University)
Tsukasa Kusakizako, Assistant Professor, Department of Biological Sciences
Keitaro Yamashita, Assistant Professor, Department of Biological Sciences / Postdoctoral Researcher, MRC Institute for Molecular Biology (current affiliation)
Satoshi Ogasawara (Project Associate Professor, Chiba University)
Takeshi Murata (Professor, Chiba University)
Tomohiro Nishizawa (Associate Professor, Department of Biological Sciences / Professor, Yokohama City University)
Osamu Nureki (Professor, Department of Biological Sciences)
Key points of the presentation
- We have succeeded in clarifying the steric structure of ATP13A2 (Note 3), which transports polyamines (Note 2) from the lumen of lysosome (Note 1) into the cell.
- By capturing the steric structures of polyamines in various states during transport, we succeeded in elucidating the polyamine transport mechanism in detail.
- Since hereditary mutations in ATP13A2 are involved in Parkinson's disease, it is expected to lead to a better understanding of these diseases.
Summary of the Announcement
Polyamines are essential compounds in living organisms, and among them, spermine, a type of polyamine, plays important functions such as activation of transcription and protection of cells from heavy metals and reactive oxygen species (Note 4). After being transported into the lysosomal lumen by endocytosis (Note 5), spermine is incorporated into the cytosolic side by ATP13A2, one of the P-type ATPases (Note 6). However, the substrate recognition and transport mechanisms of ATP13A2, which transports spermine, have not been clarified.
In this study, Professor Nureki's group at the Graduate School of Science, The University of Tokyo, and his colleagues have clarified the three-dimensional structure of human ATP13A2 by single-particle analysis method (Note 8) using cryo-EM (Note 7). They also succeeded in revealing the three-dimensional structure of ATP13A2 in multiple states, capturing the process by which ATP13A2 utilizes ATP to transport spermine, which creates a long tunnel-like pocket on the lysosomal lumen side, where spermine is widely recognized in accordance with its elongated molecular shape and and that spermine is transported along this tunnel-like pocket. This substrate recognition and transport mechanism is a novel finding and is expected to lead to further understanding of diseases caused by mutations in ATP13A2.
The research results were published online in the U.S. scientific journal Molecular Cell at 1:00 a.m. on Friday, November 19 (Japan time).
Publication details
Polyamines are essential compounds in living organisms, and spermine, a type of polyamine, plays an important role in activating transcription and protecting cells from heavy metals and reactive oxygen species. Intracellular spermine is supplied by the biosynthesis pathway and the uptake pathway from outside the cell. However, since the biosynthesis of spermine decreases with age, uptake of spermine from outside the cell is thought to be important for long-term intracellular supply of spermine. In the extracellular uptake pathway, spermine is taken up into the lysosomal lumen by endocytosis and then into the cytosol by ATP13A2, a P-type ATPase (Figure 1), ATP13A2, also known as PARK9, is one of the causative genes of Parkinson's disease. Therefore, understanding its substrate recognition and transport mechanisms is expected to lead to the establishment of methods for understanding and treating Parkinson's disease caused by hereditary mutations in ATP13A2.
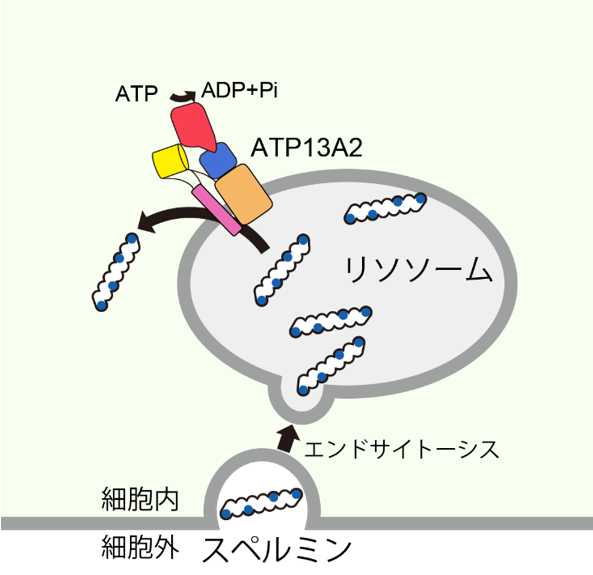
Figure 1: Spermine uptake into cells by ATP13A2
P-type ATPases have been studied in detail regarding the transport mechanisms of P2-ATPases that transport metal ions as substrates, such as sarcoplasmic reticulum Ca2+ pump (SERCA) and Na+/K+-ATPase, and P4-ATPase, a lipid flipperase P2-ATPase and P4-ATPase, a lipid flipperase, have been studied in detail. However, the mechanism by which ATP13A2 recognizes and transports spermine, a large hydrophilic molecule, has remained unclear because its chemical properties are very different from those of metal ions and lipids.
In this study, Professor Osamu Nureki and his research group at the Graduate School of Science, The University of Tokyo, determined the steric structure of human ATP13A2 in the spermine-bound state using a single particle analysis method based on cryo-EM (Figure 2).
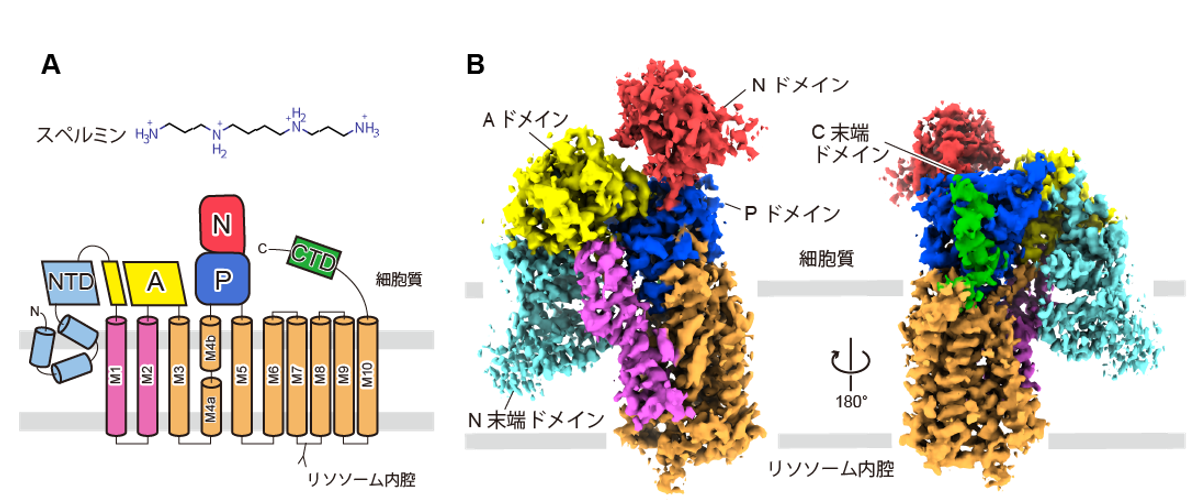
Figure 2: Chemical structure of spermine and topology of ATP13A2 (A) and cryo-EM structure (B)
The obtained three-dimensional structure showed that ATP13A2 exhibited a P-type ATPase structure and was composed of three cytoplasmic domains (A: actuator domain, N: nucleotide-binding domain, and P: phosphorylation domain) and 10 transmembrane helices (M1~10). In addition, ATP13A2 had characteristic N-terminal and C-terminal domains that are not conserved in other P-type ATPases.
In the resulting conformation, spermine bound to a substrate-binding pocket consisting of the M1-M6 transmembrane helix (Fig. 3). The substrate binding pocket had a tunnel shape composed of acidic and aromatic amino acids, and widely recognized the positively charged amino group of spermine through electrostatic interaction by the acidic amino acids and π-cation interaction (Note 9) by the aromatic amino acids. This recognition mode, consisting of a large substrate-binding pocket, is characteristic of other P-type ATPases that use small metal ions or lipids as substrates and was found to be important for spermine recognition.
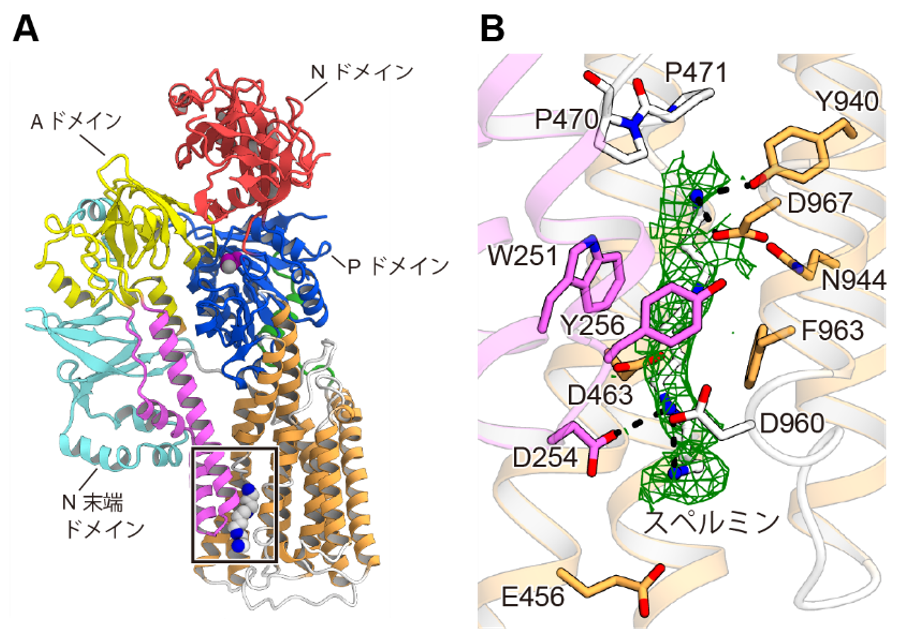
Figure 3: Spermine Recognition
(A) Three-dimensional structure of ATP13A2 in the spermine-bound state. The spermine binding site is indicated by a square. (B) Enlarged view of the spermine binding site. The density map derived from bound spermine is shown in green. Amino acid residues involved in spermine recognition are shown.
Furthermore, by combining the steric structures of several intermediates revealed using an ATP analog (AMPPCP) that mimics the ATP-binding state and aluminum fluoride ( AlF4- ) and beryllium fluoride ( BeF3- ) that mimic the phosphorylation state, and molecular dynamics simulations (Note 10 ) and the transport mechanism of spermine in ATP13A2 (Figure 4).
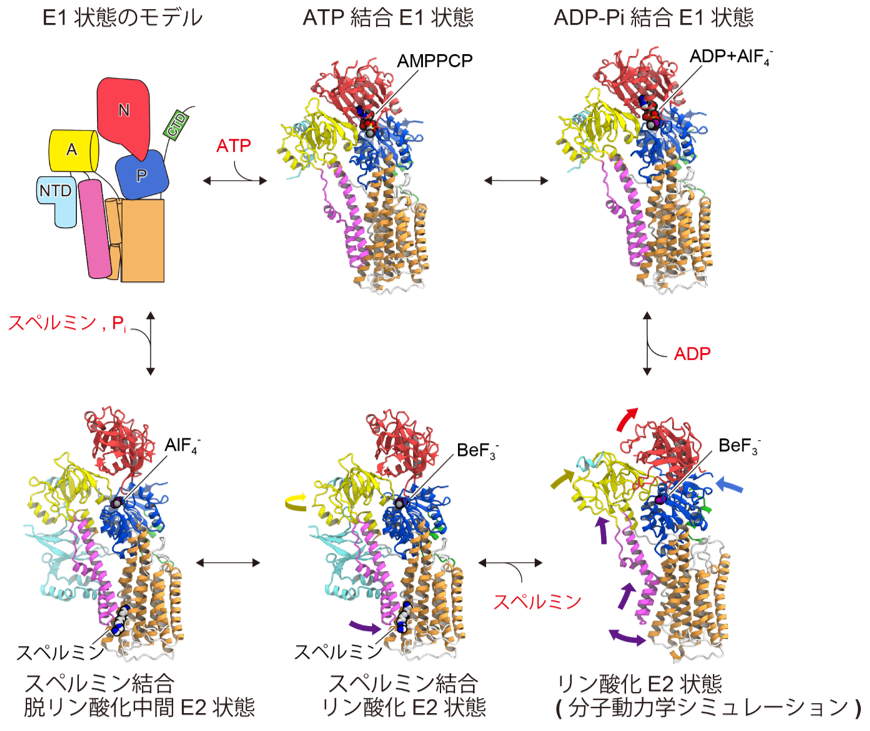
Figure 4: Spermine transport cycle of ATP13A2
The steric structure of the intermediate of ATP13A2 is shown in the spermine transport cycle.
In the phosphorylated state, ATP13A2 forms a spermine-binding pocket by large sliding of the M1 and M2 helices toward the cytoplasmic side, where spermine binds (Figures 4 and 5). Furthermore, we found that spermine binding anchors ATP13A2 in a structure that facilitates dephosphorylation. From the above, a transport mechanism was proposed in which the binding of spermine promotes the dephosphorylation reaction, and spermine is transported to the cytoplasmic side as phosphate is desorbed. Such transport of spermine is a novel mechanism and is expected to lead to further understanding of Parkinson's disease caused by mutations in ATP13A2.
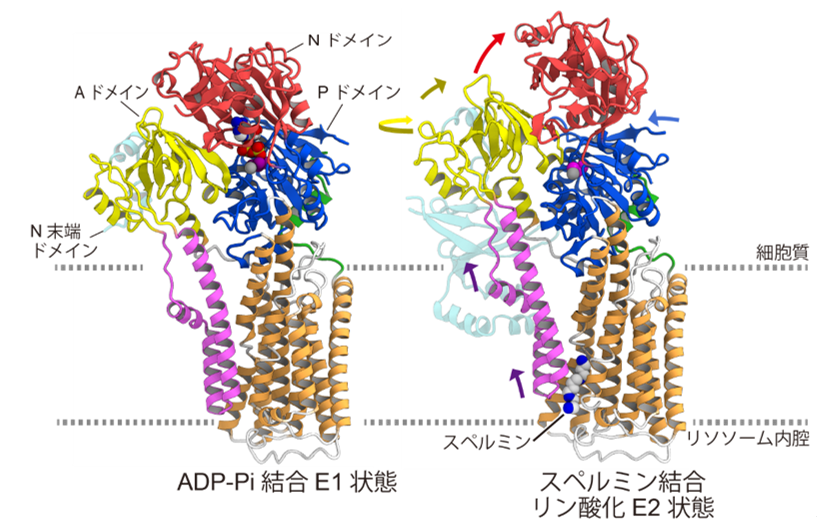
Figure 5: Structural comparison of ADP-Pi bound E1 state and spermine bound phosphorylated E2 state
This research was conducted as part of the "Elucidation of Molecular Mechanisms of Membrane Proteins Regulated by Physical Stimuli" (Research Project: 16H06294, PI: Osamu Nureki), a Grant-in-Aid for Specially Promoted Research on Science and Technology by the Japan Society for the Promotion of Science. This research was also conducted as part of the "Drug Discovery Initiative (Drug Discovery Initiative) Life Science Research Support Platform Project" of the Japan Agency for Medical Research and Development (AMED), which aims to link the results of outstanding life science research to the practical application of pharmaceuticals and other products by opening large facilities such as cryo-electron microscopes to the outside world. The project was supported by the Platform for Advanced Technology Support (BINDS).
Journals
-
Journal name Molecular CellTitle of paper Cryo-EM reveals Mechanistic Insights into Lipid-facilitated Polyamine Export by Human ATP13A2Author(s) Atsuhiro Tomita, Takashi Daiho, Tsukasa Kusakizako, Keitaro Yamashita, Satoshi Ogasawara, Takeshi Murata, Tomohiro Nishizawa* & Osamu Nureki* (author)DOI Number 10.1016/j.molcel.2021.11.001Abstract URL
Terminology
Note 1 Lysosome
A type of intracellular organelle that accumulates and degrades substances inside and outside the cell. ↑up
Note 2 Polyamine
A generic term for linear aliphatic hydrocarbons with two or more primary amino groups. They perform physiological functions when their regularly arranged positively charged amino groups interact with chemical substances such as proteins, nucleic acids, and metal ions. Spermine, spermidine, and putrescine are known as representative polyamines. ↑up
Note 3 ATP13A2
ATP13A2 is a P-type ATPase, also known as PARK9 because it is one of the genes responsible for Parkinson's disease, and was thought to transport substrates by conjugating with ATP hydrolysis, but the substrates it transported were unknown for many years. In 2020, it was reported that ATP transports polyamines such as spermine as substrates. ↑up
4 Reactive oxygen species
ROS is a generic term for a group of reactive molecules derived from oxygen molecules and produced as a byproduct of the mitochondrial electron transport system. ROS present in living organisms cause nonspecific chemical reactions to various substances and are known to be harmful to living organisms. ↑up
Note 5 Endocytosis
A process in which cells take in substances from outside the cell through phagocytosis and drinking. For substances to be finally taken up into the cytoplasm, they must be transported across the biological membrane by membrane transport proteins. ↑up
Note 6 P-type ATPase
A class of ATPases in which aspartic acid involved in enzymatic activity is phosphorylated in the process of ATP hydrolysis, resulting in a phosphorylated intermediate. Mediating the transition between two intermediate states (E1 and E2) with different affinities allows substrate transport across biological membranes. ↑up
Note 7 Cryo-electron microscopy
A device used to observe samples by irradiating electron beams on biomolecules such as proteins under liquid nitrogen (-196°C) cooling. As a method for determining the three-dimensional structure of proteins at high resolution, it has achieved remarkable technological innovation in detectors, etc. In 2017, the Nobel Prize in Chemistry was awarded to three overseas researchers who contributed to its development. ↑up
Note 8 Single particle analysis method
A method for determining the three-dimensional structure of proteins and other biological macromolecules by reconstructing the three-dimensional structure of a protein from images of a large number of biological macromolecules taken using an electron microscope. ↑up
Note 9 π-cation interaction
This is a non-covalent intermolecular interaction between a pi-electron system with abundant electrons and a neighboring cation, and the strength of the interaction is comparable to that of hydrogen bonds and salt bridges. In vivo, π-cation interactions between aromatic amino acids such as tryptophan and tyrosine, which have π-electron systems, and positively charged metal ions and amino groups are known to contribute to substrate recognition and the formation of higher-order structures. ↑up
Note 10 Molecular dynamics simulation
A method to analyze the dynamic processes (dynamics) of a system containing many atoms and molecules by solving Newton's equations in classical mechanics. When applied to the structure of a protein, it is possible to investigate how the structure changes in an aqueous solution or cell membrane. ↑up


