DATE2021.11.10 #Press Releases
Achievement of Sequential Synthesis of Optically Active Compounds Using Heterogeneous Chiral Lewis Acid Catalysts
Disclaimer: machine translated by DeepL which may contain errors.
Osamu Kobayashi, Professor, Department of Chemistry
Key points of the presentation
- We have succeeded in developing a versatile and efficient immobilization method for the chiral Lewis acid catalyst (Note 1), which is widely used in asymmetric catalytic reactions in organic synthesis.
- We immobilized a typical Lewis acid catalyst, scandium trifluoromethanesulfonate complex, and found that the prepared catalyst has high activity and selectivity for the Friedel-Crafts reaction (Note 2 ) under continuous flow conditions.
- The results of this research not only realized highly efficient stereoselective carbon-carbon bond formation in continuous flow reactions, but also are expected to be a general-purpose immobilization method that can be applied to other chiral metal catalysts.
Summary of Presentations
The enantioselective carbon-carbon bond formation reaction using chiral Lewis acid catalysts by the continuous flow method (Note 3 ) is an efficient synthetic method to form the carbon skeleton of optically active compounds (Note 4). In particular, the use of heterogeneous catalysts (Note 5) as catalysts is an ideal synthetic method because the metal catalysts can be separated and reused. Therefore, the development of a highly efficient immobilization method for solvent-soluble chiral Lewis acid catalysts was an important research issue.
Professor Osamu Kobayashi and his research group at the Graduate School of Science, The University of Tokyo, have developed a method for immobilizing scandium trifluoromethanesulfonate complexes, which are known as representative highly active Lewis acid catalysts, without any chemical modification. The heterogeneous catalyst promoted enantioselective Friedel-Crafts reaction with isatin and indole as substrates under continuous flow conditions with high purity. The catalyst also enabled continuous synthesis for more than 19 hours and achieved a higher catalytic turnover rate than the homogeneous catalyst.
This method is a versatile method that can be used to immobilize scandium complexes with various chiral ligands by a common method, and the catalyst structure can be easily tuned to the substrate structure, thus realizing the reaction with unprotected indole as a substrate, which is highly challenging.
The research results were published in the online bulletin of the German chemistry journal Angewandte Chemie International Edition on October 18 (Japan time). It was also highlighted as a particularly highly rated research result in Angewandte Chemie and published in ChemistryViews on November 9.
Publication details
Research Background
Most active pharmaceutical ingredients and natural compounds are optically active compounds, and the establishment of efficient synthetic methods is an important research issue. In 2011, the U.S. Food and Drug Administration (FDA) announced that in the next 25 years, the production of pharmaceuticals will be shifted from batch methods to continuous flow synthesis. In 2011, the U.S. Food and Drug Administration (FDA) recommended that in the next 25 years, the batch method should be replaced by the continuous flow method. In particular, flow reactions using heterogeneous catalysts are ideal for synthesis because they allow for the separation and reuse of catalyst species.
On the other hand, chiral Lewis acid-catalyzed enantioselective carbon-carbon bond formation is an important method that has been studied for more than 30 years as an efficient carbon skeleton building method for optically active compounds. Various chiral Lewis acid catalysts have been developed and a wide variety of reaction formats have been realized. However, most of the catalysts developed so far are homogeneous catalysts that are soluble in solvents and are decomposed and discarded after the reaction. Therefore, the development of chiral heterogeneous catalysts that can be used continuously in the flow method has been strongly desired.
Description of Research
In this study, a non-covalent immobilization method of scandium chiral trifluoromethanesulfonate complexes was developed using a silica surface chemically modified solid as a support. That is, by supporting a heteropoly acid, which functions as the counter anion of the scandium complex, on amine-modified silica via acid-base interaction, the cationic scandium complex was found to be firmly immobilized on the support via electrostatic interaction. The prepared catalyst was analyzed by electron microscopy and nitrogen adsorption/desorption isotherm measurements, and the structure was confirmed to be as expected (Figure 1).
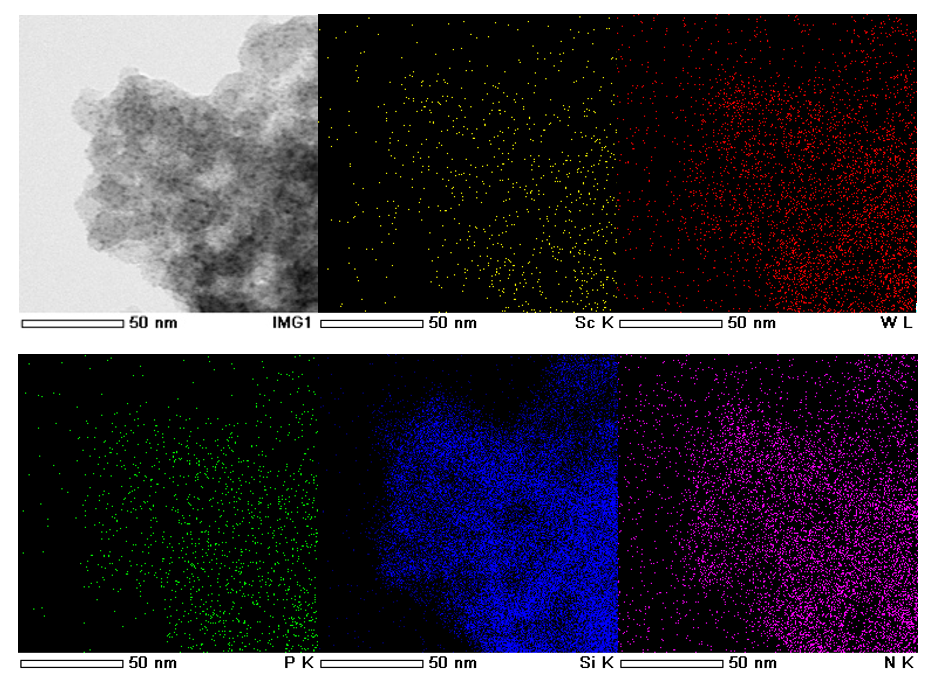
Figure 1: Elemental mapping of the catalyst by electron microscopy, showing the highly dispersed and homogeneous solid-state distribution of scandium heteropolyacid without forming aggregates.
A catalyst cartridge was prepared by packing the obtained heterogeneous catalyst into a cylindrical column, and the Friedel-Crafts reaction with isatin and indole as substrates was investigated under continuous flow conditions (Figure 2).
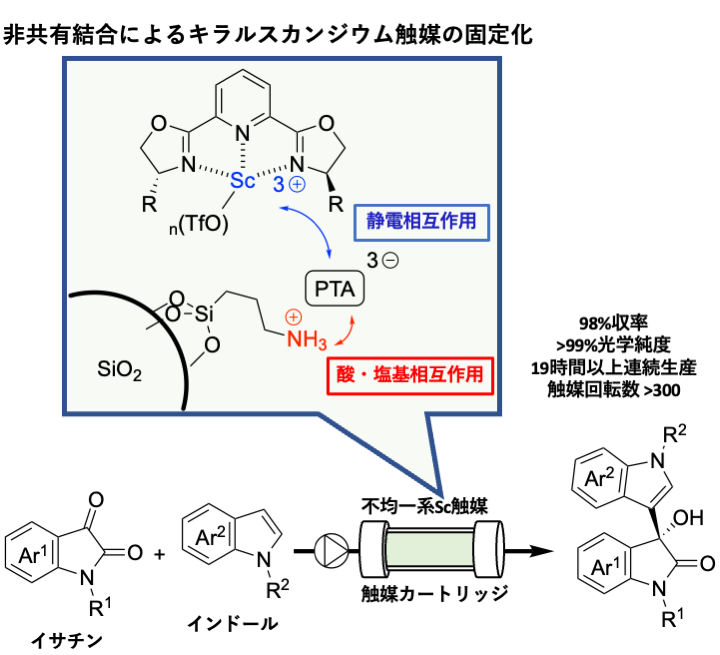
Figure 2: Continuous flow Friedel-Crafts reaction with heterogeneous chiral scandium catalyst.
It was found that the structure of the heteropoly acid, which acts as the counter anion in this scandium catalyst, has a significant effect on the activity and selectivity of the catalyst. As a result of optimization of the catalyst structure, the desired adduct was successfully obtained continuously in up to 98% yield and >99% optical purity with complete suppression of catalytic species elution. The catalytic system has a broad substrate generality and can be applied to isatin indoles with various substituents. Furthermore, since this immobilization method allows easy tuning of the ligand of the catalyst, we have found a new ligand that can yield the target compound highly selectively by optimizing the structure of the ligand in the reaction using unprotected indole as a substrate, which is highly difficult to achieve.
Future Developments
In this study, we have successfully developed an immobilization method of scandium complexes for continuous flow Lewis acid catalyzed reactions. Since the key to this method is the immobilization of cationic metal complexes by electrostatic interaction, it is expected that this method will be applied to various metal catalyst species in the future to realize continuous flow enantioselective reactions with a wide variety of chiral heterogeneous catalysts, not only Lewis acid catalysts.
Journal
-
Journal name Angewandte Chemie International EditionTitle of Paper Chiral Heterogeneous Scandium Lewis Acid Catalysts for Continuous-Flow Enantioselective Friedel-Crafts Carbon-Carbon Bond-Forming ReactionsAuthors Yuki Saito and Shū Kobayashi *DOI Number
Terminology
1 Chiral Lewis acid catalyst
A catalyst that can dramatically improve reactivity by accepting non-covalent electron pairs of electron-rich compounds such as carbonyl compounds, etc. Cationic metal complexes are mainly used. In particular, the use of chiral compounds as ligands for metal complexes enables enantioselective reactions by controlling the steric environment of the catalytically active species. Such catalysts make it possible to synthesize optically active molecules from a large number of achiral molecules using only a few chiral sources. ↑up
Note 2 Friedel-Crafts reaction
The Friedel-Crafts reaction is a nucleophilic addition reaction of an electron-rich aromatic compound to a carbonyl compound activated by Lewis acid to form a new carbon-carbon bond. In the past, compounds that produced more than chemically amphoteric wastes such as acid chlorides were used as reactants, but the use of highly active Lewis acid catalysts makes it possible to use reactants that do not produce by-products such as ketones. In particular, the use of chiral Lewis acids enables catalytic synthesis of optically active compounds. ↑up
Note 3: Continuous flow method
The continuous flow method is a synthesis method in which reaction materials are continuously fed into a reactor and products are continuously removed from the reactor at the same time. When heterogeneous catalysts are used, a cylindrical cartridge filled with catalyst is used as a reactor. By adjusting the pumping rate and operating time, this method can be used for synthesis of various scales, and has the advantages of space saving, high energy efficiency, and safety. ↑up
Note 4 Optically active compounds
When a molecule cannot be superimposed on its own mirror image, this is called a chiral molecule, and pairs of mirror images are classified as "right-handed" and "left-handed" types. When either type is present in excess, the compound has optical rotation, and such compounds are called optically active compounds. Since most active pharmaceutical ingredients are chiral molecules and only one isomer has the desired physiological activity, compounds with very high optical purity are required. ↑up
Note 5: Heterogeneous catalysts
Catalysts are classified into homogeneous and heterogeneous catalysts. Homogeneous catalysts are metal complexes and other catalysts that dissolve in a reaction solution, making synthesis and structural tuning easy. Heterogeneous catalysts are catalysts with active species immobilized on the solid itself or on the solid surface. In general, heterogeneous catalysts have the advantage that separation, recovery, and reuse of the catalyst can be easily achieved by filtration, while achieving high selectivity and leaching of the active species are problematic. ↑↑


