DATE2023.04.19 #Press Releases
These protein lumps might help regulate gene expression
Argonaute 2 protein, which controls the process of making proteins based on genetic code, forms lumps, and the finding might help develop methods to precisely control gene expression.
April 19, 2023
Proteins are made up of 20 different amino acids in sequence, and an end region of the amino acid sequence is called the N-terminal. Most of the known Argonaute proteins—which are important for silencing RNA and thus regulating gene expression—have a short N-terminal. But Argonaute 2 protein in fruit flies, Drosophila melanogaster, has a long and unique N-terminal region (Figure 1), whose function remained a mystery. A new study by Haruka Narita, Tomohiro Shima, and their team shows that the N-terminal region in Argonaute 2 can form aggregates, helping them better understand how to control gene expression in different organisms.
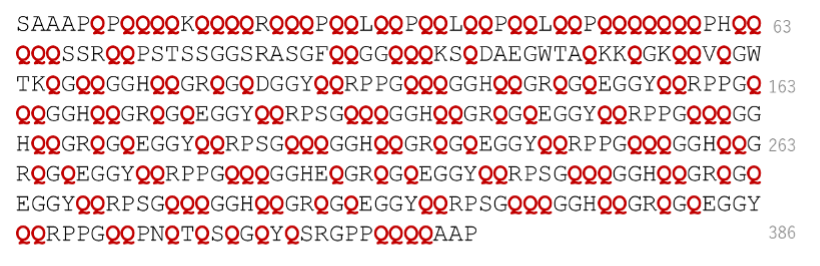
Figure 1. The amino acid sequence of the N-terminal region of Drosophila Argonaute 2. Each amino acid in a protein is usually only a few percent of the whole sequence. In the N-terminal region, however, one of the amino acids (glutamine, Q in red) makes up more than 40% of the total, while the rest of the protein contains only about 4% glutamine, highlighting the uniqueness of the N-terminal sequence. The number on the far right indicates the number of amino acid residues counting from the beginning of the Argonaute protein sequence.
“We noticed that the N-terminal region has a sequence similar to that of prions [proteins that can change the shape of other proteins into an abnormal form], which are thought to cause mad cow disease,” says Shima. Prions form insoluble aggregates or amyloids when many prion molecules tightly bind together. Such amyloid aggregates of proteins are also involved in diseases such as Alzheimer’s disease and Type 2 Diabetes. So, Shima and his team wondered whether the N-terminal region of Argonaute 2 also forms amyloid-like aggregates. They tested their prediction using three ways: detergent resistance assay, amyloid detection reagent, and microscopy.
Detergents can disrupt protein interactions, but proteins in amyloid (insoluble filamentous structures) aggregates do not dissociate even in strong detergents. To their surprise, the N-terminal region of Argonaute 2 indeed formed amyloid-like aggregates, but a majority also formed fractal-like aggregates (Figure 2). The researchers further confirmed their findings using an amyloid detection reagent that specifically binds to amyloids. They could also observe the aggregates under a microscope.
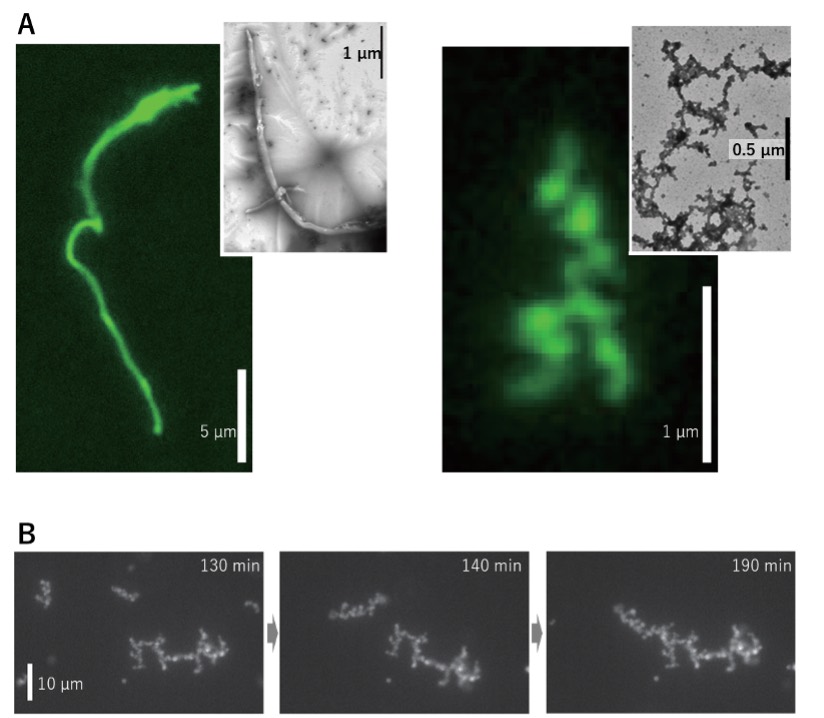
Figure 2. Microscopic images of N-terminal aggregates. (A) While some of the N-terminal aggregates showed a filamentous shape like normal amyloids (left), the majority had a different shape, called fractal-shaped aggregates (right). (B) Time-lapse images of how the fractal-shaped aggregates bind and grow. The numbers in the upper right of the panels indicate the time from the start of the aggregation process.
However, their conclusions are based on theoretical predictions and biochemical assays outside living cells. “We would also like to study more physiological aspects, such as whether aggregation and disaggregation of Argonautes actually occur in living cells, and if so, how they are involved in the regulation of gene expression,” says Shima. “Our results raise the possibility that the regulation of gene expression by Argonaute may also be more finely controlled by N-terminal aggregation.”
The Argonaute’s gene regulation ability is widely used as a fundamental tool in cell and medical biology. Like in the insect model organism Drosophila melanogaster, Argonaute in the well-known plant model called Arabidopsis thaliana also forms aggregates. Therefore, this study may help develop methods for precisely controlling gene expression in different organisms.
Publication details
Journal BMC Biology Title N-terminal region of Drosophila melanogaster Argonaute 2 forms amyloid-like aggregates AuthorsHaruka Narita, Tomohiro Shima, Ryo Iizuka, and Sotaro UemuraDOI10.1186/s12915-023-01569-3


