DATE2023.04.25 #Press Releases
The structure of the ETB-Gi complex revealed: a step toward enhanced anti-cancer drugs
Using novel techniques, UTokyo scientists revealed the structure of the endothelin-1-ETB-Gi complex that controls blood flow in our body.
April 25, 2023
Peptides called endothelins (ET) help control the blood flow in our body and affect various cellular processes. In the epithelial cell membrane, an endothelin receptor called ETB induces the relaxation of blood vessels as it can activate many types of G-proteins within the cell. Hence, ETB antagonists have the potential for enhanced anti-tumor drug delivery, among other pharmacological applications. Understanding the activation of ETB and the structure of ETB-G protein complexes is thus vital for drug discovery. In a study published in the journal eLife, UTokyo scientists led by Wataru Shihoya and Osamu Nureki revealed the structure of an endothelin-1-ETB-Gi complex at high resolution using cryo-electron microscopy.
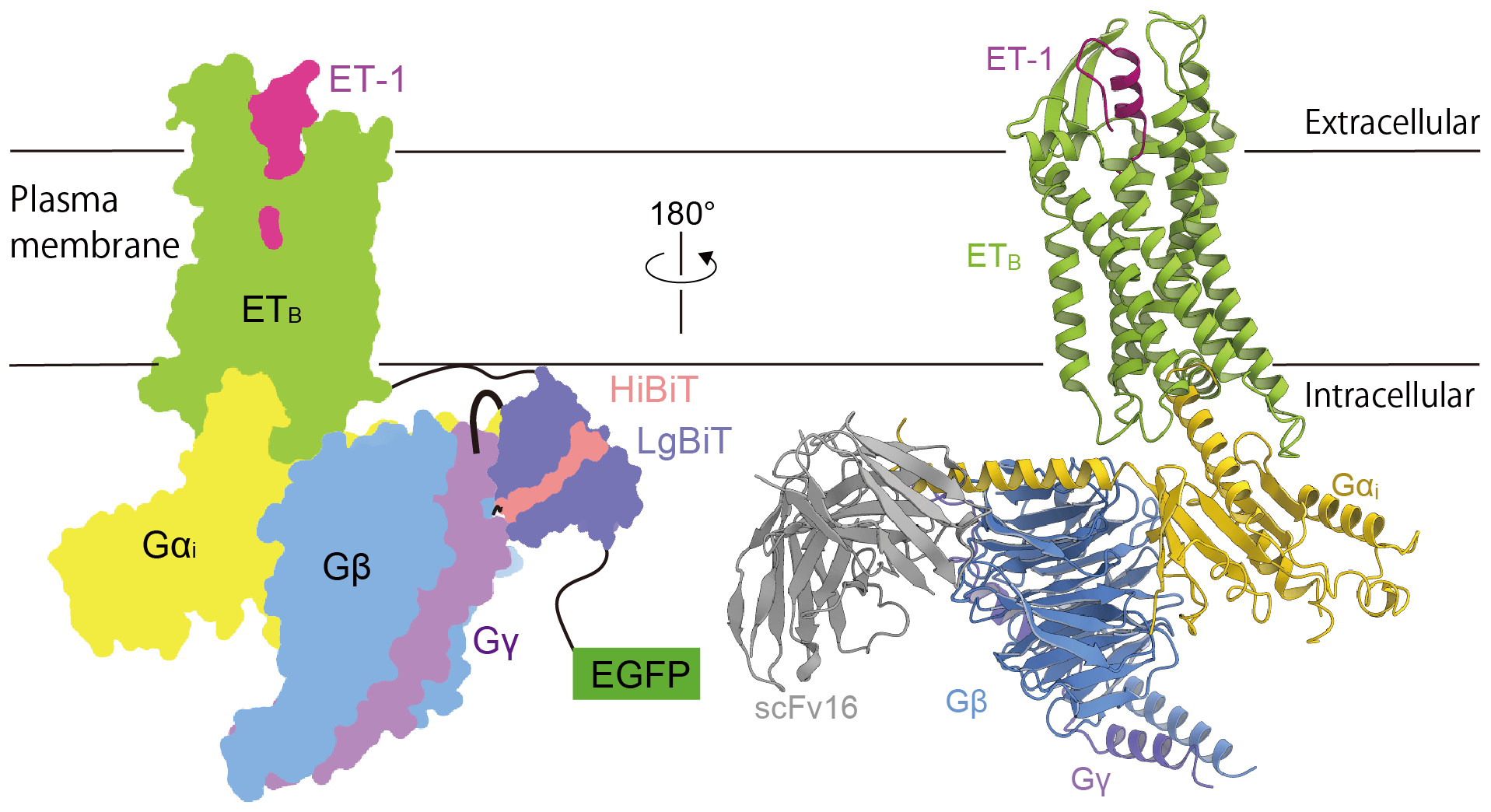
Figure 1. Schematic representation and structure of the endothelin-1-ETB-Gi complex.
In the cryo-electron microscopy technique, scientists freeze proteins up to -196° C using liquid nitrogen and irradiate them with electron beams, which reveals their 3D structure. “The freezing helps keep the proteins in their natural shape and stops them from getting damaged while we study them,” explains Wataru Shihoya, an assistant professor at UTokyo and one of the authors. This method helps them study how proteins interact with each other, aiding the development of new medicines.
Shihoya has been working on ETB research since he was an undergraduate and has published various X-ray crystal structures of ETB bound to different drugs. But, the ETB and G-protein complex structure remained unsolved, and the G-protein activation mechanism has been a long-standing mystery. Shihoya feels a sense of accomplishment now as he has been able to bring closure to this research, which he inherited from his late mentor.
To study the ETB-Gi complex, the research team stabilized the molecule using a new method called the ‘Fusion-G system’. This system allowed them to purify and obtain a stable endothelin-1-ETB-Gi complex to study. “By examining this complex, we discovered unique aspects of how the ETB receptor gets activated by the agonist endothelin-1 and interacts with Gi,” says Shihoya. They found that the ETB receptor activates differently than similar receptors and binds to the Gi protein in a special way. This new information will help in drug designs that work better on this receptor and efficiently treat conditions such as high blood pressure, heart disease, and brain damage.
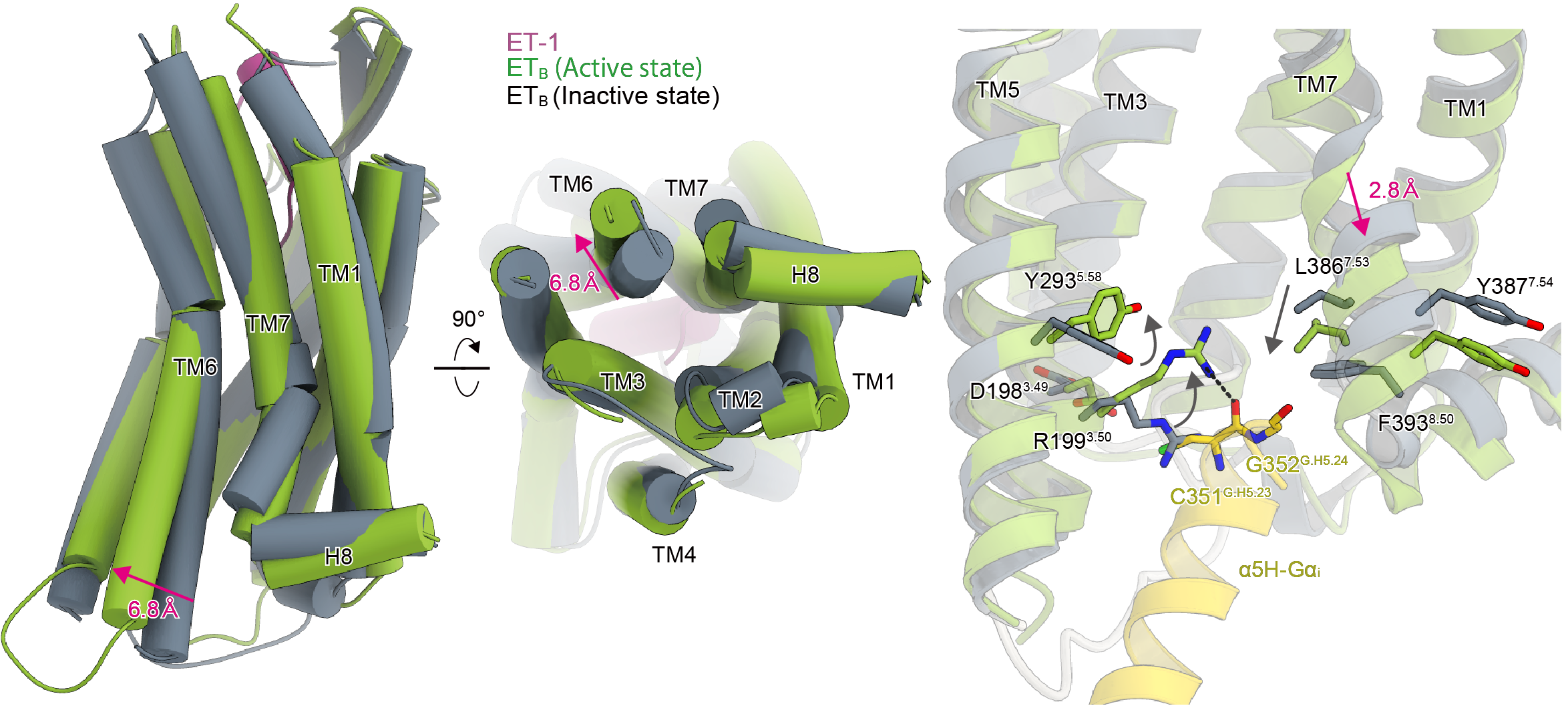
Figure 2. Conformational changes on receptor activation. The inactive and active ETB receptors are in grey and green, respectively. Upon endothelin-1 binding, as indicated by the arrow in the figure, the intracellular side of the receptor opens to allow interaction with the Gi protein.
Shihoya is excited about the future possibilities. “We want to investigate the unique activation mechanism of the ETB receptor further and explore how we can target it for the development of small molecule drugs that can selectively activate the ETB receptor,” he says. “Additionally, our ‘Fusion-G system’ can be used to study other receptor-protein complexes, advancing our knowledge in various areas of biology and pharmacology.”
Publication details
Journal eLife Title Cryo-EM structure of the endothelin-1-ETB-Gi complex AuthorsFumiya K Sano, Hiroaki Akasaka, Wataru Shihoya, Osamu NurekiDOI


