DATE2023.03.07 #Press Releases
Mechanism of resolution of the collision ribosome, the entity of abnormal translation
Disclaimer: machine translated by DeepL which may contain errors.
--Aberrant translation resolution mechanism by ribosomal conformational change due to mRNA pulling force.
The University of Tokyo Institute of Medical Science
Graduate School of Science, The University of Tokyo
Graduate School of Frontier Sciences, The University of Tokyo
Summary of Presentations
A research group led by Professor Toshifumi Inada, Department of Computational Biology and Medical Sciences, Institute of Medical Science, The University of Tokyo, Associate Professor Yoshitaka Matsuo, Department of Computational Biology and Medical Sciences, Graduate School of Biological Sciences, Graduate School of Science, and Department of Frontier Sciences, The University of Tokyo, and graduate student Katharina Best and Professor Roland Beckmann's group was the first in the world to elucidate that the translation factor eIF5A promotes an atypical translation CAT tailing response.
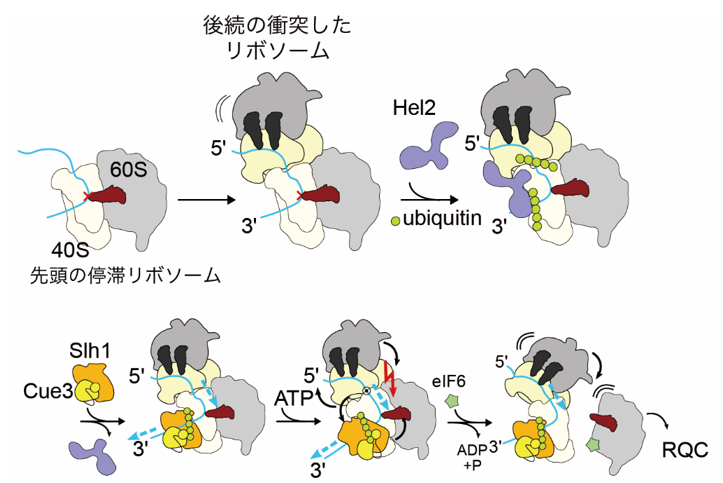
When ribosomal traffic congestion accumulates due to translation stagnation, various stress responses such as cell death and inflammatory responses are induced. On the other hand, to prevent excessive stress responses, cells have a quality control mechanism that resolves ribosomal traffic jams. Hel2, a quality control factor, identifies ribosome collisions and adds ubiquitin as a marker for abnormal translation. This ubiquitination serves as a marker and the RQT (Ribosome Quality control Trigger ) complex resolves traffic jams by forcing subunit dissociation of stagnant ribosomes. The research group has previously used biochemical methods to discover that K63-type ubiquitin chains are formed on collision ribosomes, which are identified by the RQT complex and ATP hydrolysis-dependent dissociation of collision ribosomes. On the other hand, the molecular mechanism of ATP hydrolysis-dependent dissociation of collision ribosomes into their respective subunits was not fully understood.
In this study, the research group successfully visualized the collision ribosome-resolving complex (RQT complex) and collision ribosomes using cryo-electron microscopy. As a result, they revealed for the first time in the world that the RQT complex applies a tensile force to mRNA, destabilizes the structural changes of the small subunits of the ribosome, and ultimately dissociates the subunits.
This achievement is expected to lead to a better understanding of the pathogenic mechanism of neurodegenerative diseases, such as those caused by a breakdown of the quality control mechanism, and to the development of novel therapeutic strategies.
The research results were published in the U.S. scientific journal Nature Communications on February 17.
For more information, please visit the website of the Institute of Medical Science, The University of Tokyo.


