DATE2022.12.21 #Press Releases
Cells rearrange to shape a tissue during development
Thanks to the interplay of geometry, mechanics, and molecular signaling at the cell junctions.
December 21, 2022
When you remodel a house, rearranging the individual building blocks is often necessary. Epithelial cells do the same when shaping a tissue. A team of researchers led by Kaoru Sugimura from the University of Tokyo reveals how epithelial cells rearrange in the wings of a fruit fly, Drosophila melanogaster. Specifically, they show the role of the temporary formation of a subcellular structure called rectangular-shaped Myosin-II Cables (rsMC) during the process. Their findings appear in the latest issue of the journal Current Biology.
Cells are like bricks. Cement binds bricks; extracellular matrix and cell adhesion bind cells. They stick to each other as specialized surface molecules form cell junctions. During tissue development, the cells push and pull each other to rearrange themselves. The rearrangement happens in three steps (Figure 1 , Video 1). First, a cell junction shrinks to bring two different cells closer. Then the cells exchange junctions around the vertices of cells. Finally, the newly generated cell junction elongates and thus completes the cell rearrangement. Researchers know the molecular mechanisms of cell junction shrinkage and elongation. But they knew little about how cells exchange junctions. Associate Professor Sugimura’s team reveals the molecular mystery behind cell junction exchange.
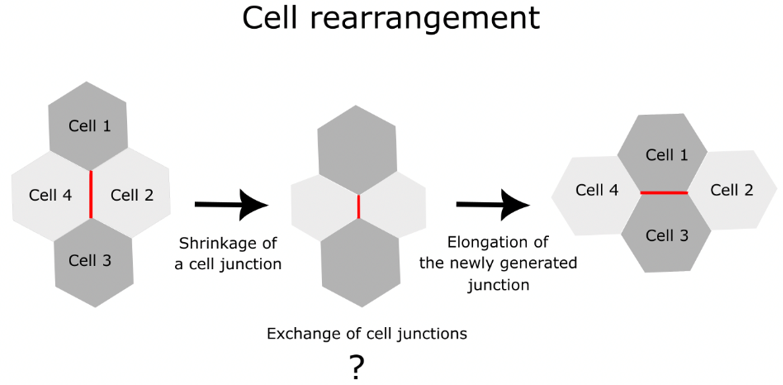
Figure 1 : Cell rearrangement process. The red line shows the cell junctions involved in the rearrangement process. This study revealed the molecular and mechanical processes involved in the exchange of cell junctions.
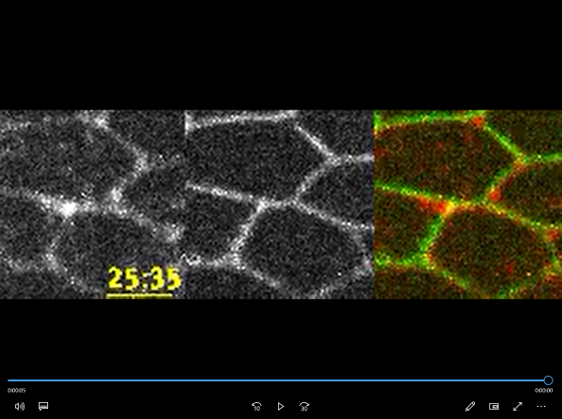
Video 1 :Time-lapse images of myo-II-mKate2, a marker for Myosin-II Cables (gray in the left panels, red in the right panel), during cell rearrangement in the Drosophila melanogaster pupal wing. A transmembrane protein called DE-cadherin tagged with the green fluorescent protein (GFP) is shown in gray in the left panels and green in the right panel.
They used mechanical simulations and imaging techniques to understand cell junction exchange. The imaging helped them visualize the distribution of various molecules during the process. During cell junction exchange, rectangular-shaped Myosin-II Cables (rsMC) formed for a short duration. On average, around 4.3 minutes later, the rsMC breaks up, leading to the formation of a newly generated junction (Video 1, Figure 2). Imaging techniques further revealed that an antagonistic interaction of proteins called Jub and M6 regulates the formation and breaking of rsMC.
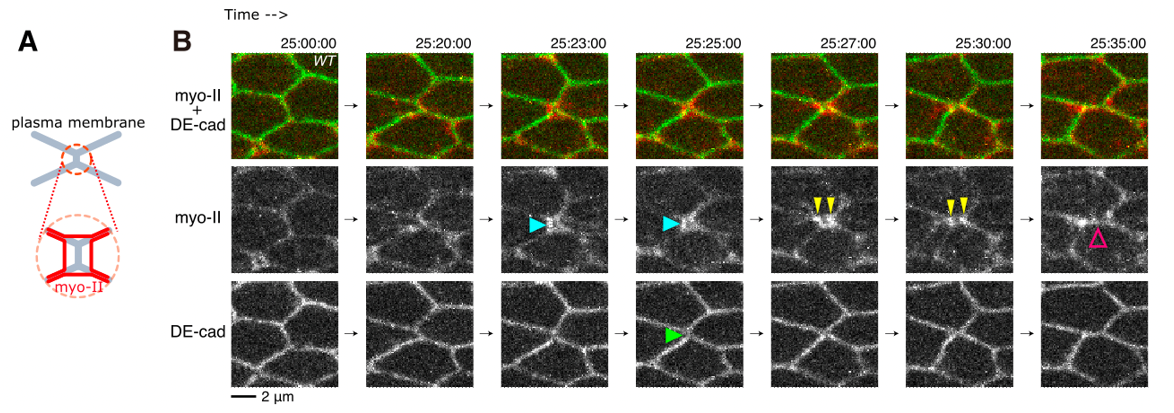
Figure 2 : Cell junction exchange process. (A) Schematics of the cellular structures studied here. The orange circle shows the zoomed-in schematic of the region of interest. The gray lines are the plasma membranes, and the red lines are myosin-II. (B) Time-lapse images as seen in Video 1. The top panel shows both myo-II and DE-cad-GFP, while the bottom panels visualize myo-II and DE-cad separately. Blue, yellow, and magenta arrows show the detachment of myo-II from the junction, the separation of myo-II signals, and the formation of a new junction. A green arrow points to the DE-cad signal inside the rsMC.
The researchers then set out to understand the physical basis of the junction exchange process. They constructed a mechanical model of the rsMC before, during, and after the junction exchange process. The model uncovered the mechanical and geometrical conditions that allow the attachment and detachment of Myosin-II Cables from the cell junctions. The researchers conclude that the cell junction exchange happens due to an interplay of geometry, mechanics, and molecular signaling.
The team has revealed the molecular mechanism of cell junction exchange in a developing fruit fly. But the findings have implications for other animals as well. “Cell adhesion structure is ubiquitous in all organisms and important for tissue morphogenesis [the process by which tissues and organs develop their shape] and homeostasis [maintenance of a stable internal state], cancer progression, and aging,” says Keisuke Ikawa, the first author of the research article. “The uncovered mechanism in this study might be relevant to these biological and lifecycle events.”
Publication details
Journal Current Biology Title Attachment and detachment of cortical myosin regulates cell junction exchange during cell rearrangement in the Drosophila wing epitheliumAuthors Keisuke Ikawa, Shuji Ishihara, Yoichiro Tamori, and Kaoru SugimuraDOI


