DATE2022.08.09 #Press Releases
Visualization of the PTH1 receptor signaling complex involved in bone metabolism
Disclaimer: machine translated by DeepL which may contain errors.
--Contributing to the rational design of safe and effective osteoporosis drugs
Kazuhiro Kobayashi (Doctoral Student, Department of Biological Sciences)
Koki Kawakami (Assistant Professor, Graduate School of Pharmacy, Tohoku University)
Tsukasa Kusakizako (Assistant Professor, Department of Biological Sciences)
Hideaki Kato (Associate Professor, Graduate School of Arts and Sciences, Komaba Institute for Science and Department of Biological Sciences, The University of Tokyo)
Asuka Inoue (Professor, Graduate School of Pharmacy, Tohoku University)
Osamu Nureki (Professor, Department of Biological Sciences)
Key points of the presentation
- Using cryo-EM, we have visualized the signaling complex structure of the parathyroid hormone type 1 receptor (PTH1 receptor), which plays a central role in bone metabolism, in which two hormone molecules with different actions (parathyroid hormone (PTH) and parathyroid hormone-related peptide (PTHrP)) are bound. The structure of the signal transduction complex in which PTH and PTHrP bind to each other was visualized.
- We found that PTH and PTHrP bind to their receptors in different modes, revealing the structural basis that defines the differences in physiological responses.
- We revealed the sequential structural changes in the process of PTH desorption from the PTH1 receptor and proposed an activation cycle of the PTH1 receptor.
Summary of Presentation
Bone is one of the essential organs in humans and plays many roles in maintaining the skeleton, protecting organs, and regulating blood calcium levels. PTH (Note 1) and PTHrP (Note 2) are the most important peptides in all processes from bone formation to maintenance, and their functions are regulated by PTH1 receptor (Note 3) and G protein trimer (Note 4 ), one of the stimulatory G protein (Gs protein) trimers. While both PTH and PTHrP promote bone formation and have attracted attention as a therapeutic agent for osteoporosis, these hormones are also known to be involved in excessive PTH1 receptor-mediated Since PTH and PTHrP have different levels of side effects, it is hoped that understanding the differences in the mechanisms of action of these hormones will lead to the development of drugs with reduced side effects. However, the structural mechanism by which PTH1 receptors are activated by these hormones is unknown, and the mechanism that produces side effects has not been clarified.
In this study, Professor Nureki's group at The University of Tokyo's Graduate School of Science has elucidated the three-dimensional structure of a signaling complex consisting of the PTH1 receptor bound to PTH and PTHrP and a Gs protein trimer by cryo-EM single particle structural analysis (Note 5). PTH-bound PTH1 receptor and PTHrP-bound PTH1 receptor revealed that the two hormones form different interactions with the PTH1 receptor, and in collaboration with Professor Asuka Inoue of Graduate School of Pharmacy, Tohoku University, we identified the interaction between PTH and PTHrP that is important for the activation of the PTH1 receptor. The team has identified the following In addition, the differences in the interactions between the two hormones have revealed the mechanisms by which they produce adverse effects.
In addition, a total of five different conformational states of the PTH-bound PTH1 receptor and Gs protein complex were obtained from the samples, and computer simulation analysis revealed that these conformational states represent the conformational changes during the process of PTH release from the receptor.
The results of this study are expected to deepen our understanding of the structural basis for the differences in physiological responses between PTH and PTHrP, and to facilitate the development of osteoporosis drugs with fewer side effects.
Contents of Presentation
Background of the research and problems in previous studies
G protein-coupled receptors (GPCRs, (Note 6) ) form the largest family of proteins in humans, and the approximately 800 species of GPCRs regulate most physiological phenomena in humans. The PTH1 receptor, the subject of this study, is known as one of the most important factors in bone metabolism; its binding to two endogenous ligands (Note 7 ), PTH and PTHrP, causes a conformational change to the active form, and the activated PTH1 receptor induces bone formation. Because of these osteogenic effects, these ligands and their modified ligands are used as the main therapeutic agents for osteoporosis. However, these drugs also induce bone destruction as a side effect, and the search for new osteoporosis drugs with fewer side effects continues. In particular, PTHrP is characterized by a lower incidence of side effects than PTH, and it has been reported that the side effects of PTH and PTHrP are correlated with the length of time the receptor is activated. Therefore, the development of osteoporosis drugs with fewer side effects is long awaited by using the steric structure of the PTH1 receptor bound to PTH and PTHrP to elucidate the mechanism that controls the activation time of the receptor. However, the steric structure of the PTH1 receptor activated by these ligands has not been clarified, making it difficult to determine the control mechanism of receptor activation time.
Research Details
In this study, a research group led by Kazuhiro Kobayashi, Doctoral student, Assistant Professor Shoji Kusaki, Associate Professor Hideaki Kato, and Professor Osamu Nureki at the Graduate School of Science, The University of Tokyo, clarified the steric structure of the activated complex of the PTH1 receptor bound to PTH or PTHrP and Gs protein trimer by single particle analysis using cryo-electron emission microscopy PTH bound at its C-terminus to the extracellular domain of the PTH1 receptor (Note 8 ) and at its N-terminus to the transmembrane domain of the PTH1 receptor (Note 9), and these interactions formed a stable helix structure with almost the entire ligand length.
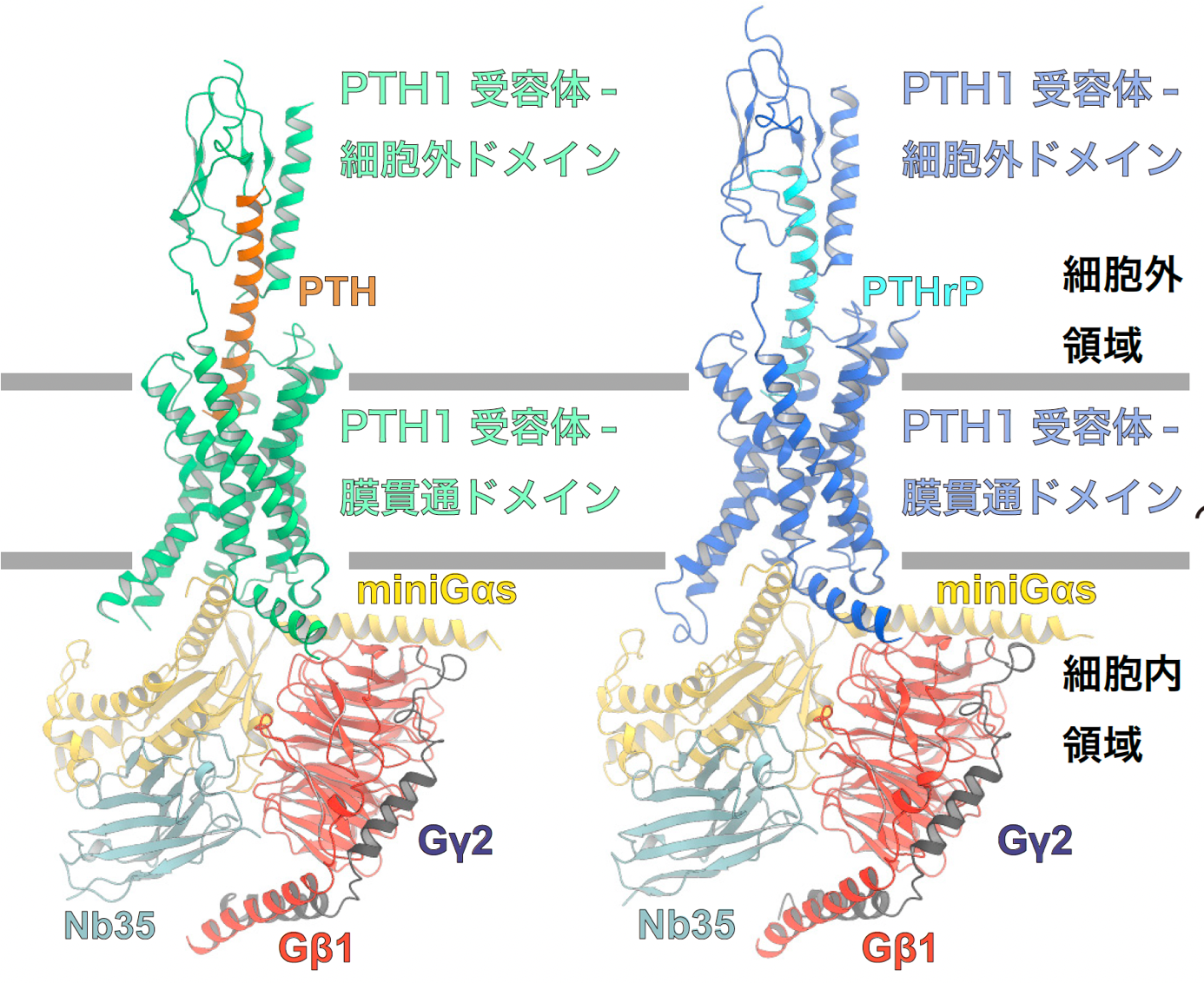
Figure 1: Overall structure of the PTH1 receptor signaling complex.
The figure shows the overall structure of the signaling complex of PTH-bound PTH1 receptor and Gs protein (left panel) and PTHrP-bound PTH1 receptor and Gs protein (right panel). In this study, the structure of Gαs was successfully determined using mini Gαs, a thermostable mutant of Gαs. In order to improve the stability of the signaling complex, an antibody that recognizes the complex structure, Nb35, was used.
PTH and PTHrP form extensive hydrophilic interactions with the transmembrane domain of the PTH1 receptor, and mutant analysis revealed that these interactions are essential for receptor activation (Figures 2 and 3). In addition, these ligands form a characteristic interaction with the hydrophobic pocket formed between the third and fifth transmembrane helices (TM3 and TM5) of the receptor (Fig. 2), and PTH forms a close interaction with this pocket, whereas PTHrP binds to the hydrophobic pocket at a different position On the other hand, PTHrP has a different amino acid sequence from PTH that is present in the position for binding to the hydrophobic pocket, and does not form sufficient binding to this hydrophobic pocket (Fig. 3). The importance of the specific interaction between the PTH1R receptor and the ligand observed by cryo-EM was demonstrated by Assistant Professor Koki Kawakami and Professor Asuka Inoue of the Graduate School of Pharmacy at Tohoku University by analyzing the signaling activity of PTH1R mutants in cultured cells. These ligands bind to this hydrophobic pocket only in the activated state, suggesting that PTH has a strong effect on keeping the receptor in the activated state, while PTHrP has a weak effect on keeping the receptor in the activated state.
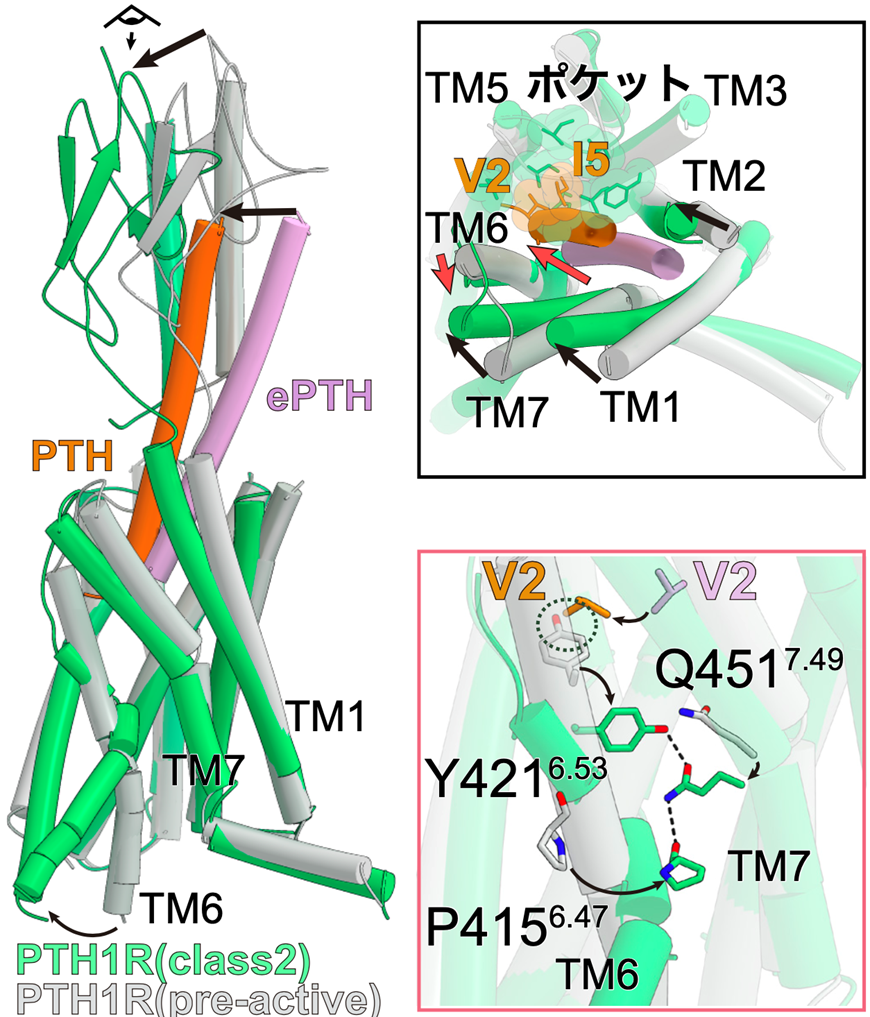
Figure 2: ePTH-bound inactivated PTH1 receptor and PTH-bound activated PTH1 receptor
Comparing the pre-activated PTH1 receptor with the activated PTH-bound PTH1 receptor, the intracellular side of TM6 in the activated structure had a large conformational change, creating space for G protein to bind (left panel). Looking at the receptor from the extracellular side, TM1, 2, and 7 of the receptor underwent conformational changes toward TM5 upon activation, while TM6 underwent conformational changes toward the outer side of the receptor (upper right panel). The conformational change of TM6 upon activation and the YQP motif (consisting of Y421, Q451, and P415) that stabilizes it are formed (bottom right figure).
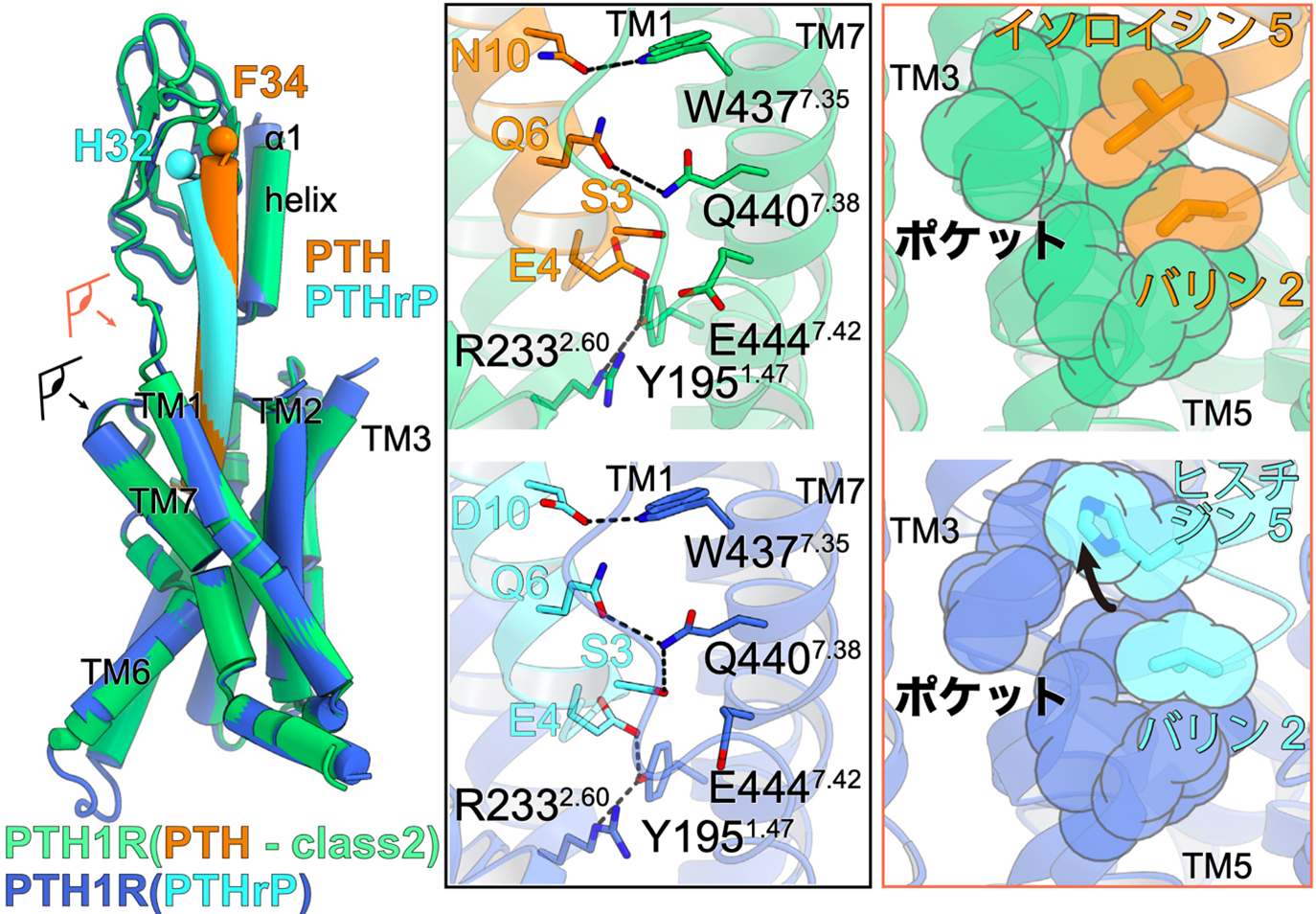
Figure 3: Ligand interactions involved in PTH1 receptor activation.
Superimposed view of PTH-bound PTH1 receptor and PTHrP-bound PTH1 receptor (left figure). The eye and arrows indicate where the figure is viewed from and correspond to the figures on the right, respectively.
Interaction between PTH (orange) and PTH1 receptor (green) on the left and PTHrP (light blue) and PTH1 receptor (blue) on the right. The second and fifth residues of the ligand interact with the hydrophobic pocket formed at the third transmembrane helix (TM3) and TM5. PTHrP, on the other hand, has a histidine residue at residue 5, which can only interact with a portion of the hydrophobic pocket.
Structural analysis of the PTH-bound PTH1 receptor also revealed five distinct structures during the ligand desorption process (Fig. 4). The obtained structures and computational simulations reveal that the inactivation of the PTH-activated PTH1 receptor is triggered by the loss of the helix structure of the ligand starting from the 17th amino acid residue of the ligand, followed by the loss of the interaction between the PTH ligand and the hydrophobic pocket of the receptor A series of conformational changes were revealed, which were followed by the loss of interaction between the PTH ligand and the hydrophobic pocket of the receptor. Understanding this conformational change will contribute to the development of drugs with reduced side effects by regulating the activation time of the receptor by modulating the binding of specific ligand amino acid residues to the receptor.
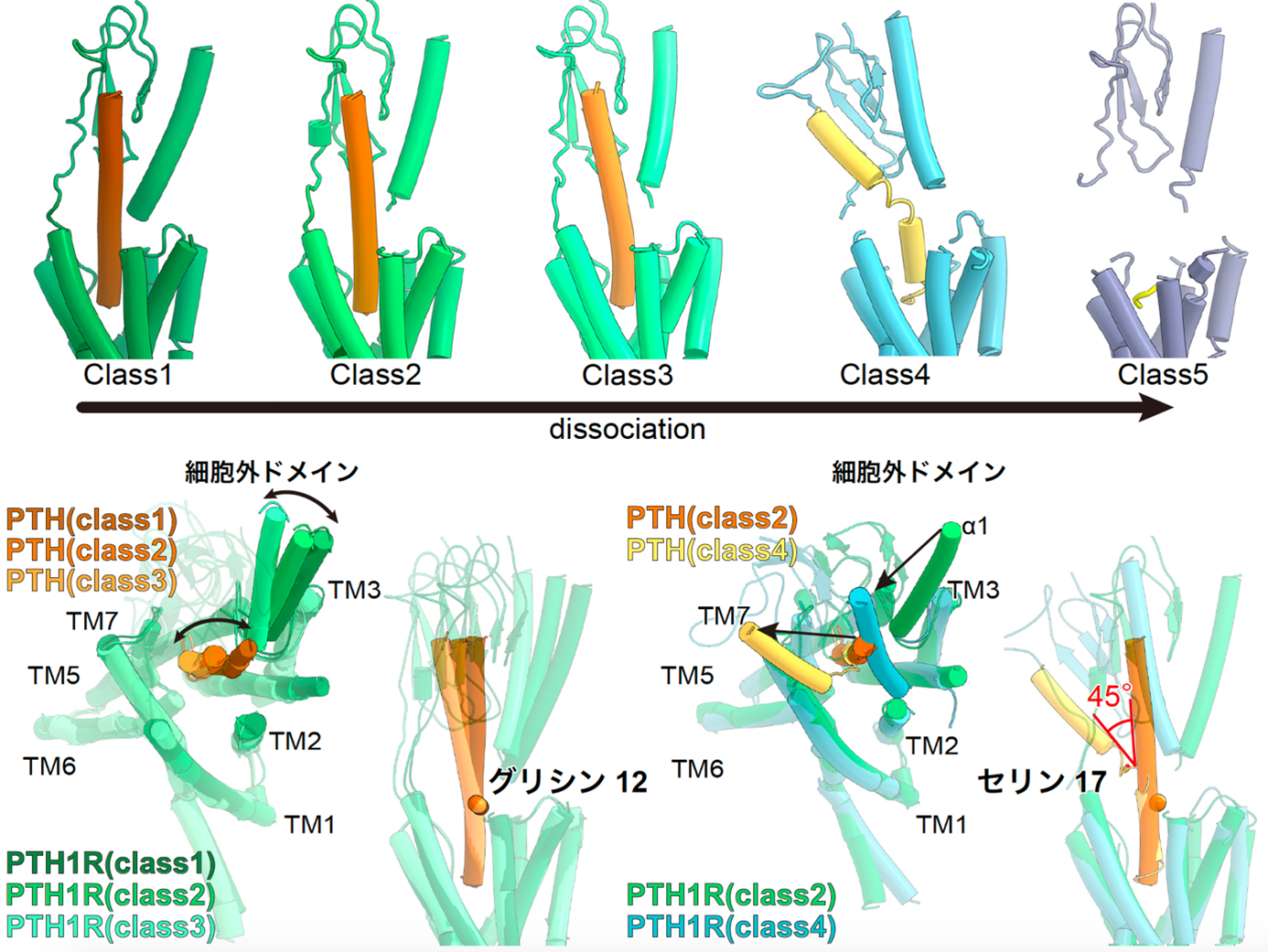
Figure 4: Five different structures of the PTH-bound PTH1 receptor and the Gs protein complex.
The PTH-bound PTH1 receptor and Gs protein complex revealed five different structures that capture the PTH shedding process (top figure). The structure of the extracellular domain and PTH change jointly starting from the glycine at residue 12 (lower left figure). On the other hand, in Class 4, PTH showed a large fold of about 45 degrees at serine residue 17, and in Class 5, PTH did not adopt a rigid structure except for the five residues at the N-terminus, which are essential for receptor activation.
In addition, these structures revealed that the PTH1 receptor loses its characteristic interaction with the Gs protein when the extracellular region is destabilized (Figure 5). These results clarify the structural basis of the PTH1 receptor-mediated adverse effect mechanism and are the first in the world to experimentally capture the structural changes during the process of ligand release from the GPCR.
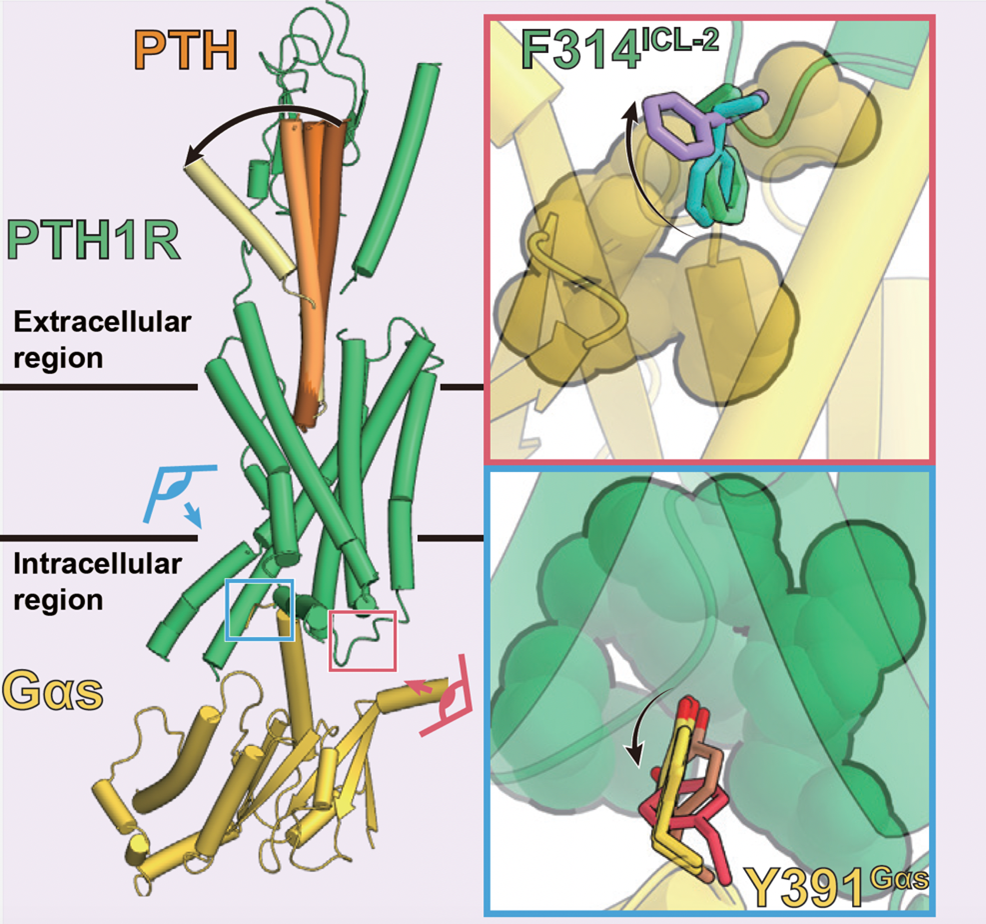
Figure 5: Sequential conformational changes of PTH ligand and transition of interaction at the PTH1 receptor-G protein interface
The multiple structures revealed by this study indicate that the characteristic interaction between the PTH1 receptor and the G protein is lost (upper right: green to purple, lower right: yellow to red) in response to the conformational change of the PTH ligand (left panel: dark brown to yellow).
Social Significance and Future Plans
This study clarified the mechanism of PTH1R-mediated side effect expression and the sequential ligand elimination process by combining steric structure analysis, computational simulation, and functional analysis using cultured cells. The results of this study are expected to be useful for rational design of osteoporosis drugs with reduced side effects.
This study was supported by Grant-in-Aid for Specially Promoted Research "Elucidation of Molecular Mechanisms of Membrane Proteins Regulated by Physical Stimuli" (Principal Investigator: Osamu Nureki) and other Grant-in-Aid for Scientific Research Projects (Project Numbers: 16H06294, 19H03163, 20J21820, 21H05142, 21H 04791, 21H05113, JPJSBP120213501, JPJSBP120218801), as well as the Drug Discovery Initiative (BINDS) (JP20am0101095), the Innovative R&D for Advanced Research and Development Solo type (PRIME) (JP19gm5910013), Incubate type (LEAP) (JP20gm0010004), Strategic Creative Research Promotion Program PRESTO (JPMJPR1331) and Moon JPMJMS2023), Takeda Science Foundation, Astellas Research Group for Pathophysiology and Metabolism, Kao Foundation for Arts and Sciences, Mochida Foundation, Tokyo Biochemical Society, Daiichi Sansei Life Science Research Foundation, Uehara Memorial Life Science Foundation, and many others.
Journals
-
Journal name Molecular Cell Title of paper Endogenous ligand recognition and structural transition of a human PTH receptor Author(s) Kazuhiro Kobayashi (1), Kouki Kawakami (2), Tsukasa Kusakizako (1), Hirotake Miyauchi (1), Atsuhiro Tomita (1), Kan Kobayashi (1), Wataru Shihoya (1), Keitaro Yamashita (1, 3), Tomohiro Nishizawa (1, 4), Hidehiro Shihoya (1) Keitaro Yamashita (1, 3), Tomohiro Nishizawa (1, 4), Hideaki E. Kato (1, 5),*, Asuka Inoue (2),*, Osamu Nureki (1)*.
*Joint Responsible Authors (1) Department of Multidisciplinary Sciences, Graduate School of Science, The University of Tokyo (2) Department of Molecular Cell Biology, Graduate School of Pharmaceutical Sciences, Tohoku University (3) MRC Research Institute (4) Graduate School of Biomedical Sciences, Yokohama City University (5) Department of Biological Sciences, Graduate School of Arts and Sciences, The University of TokyoDOI No.
Terminology
Note 1 Parathyroid hormone (PTH )
PTH is an 84 amino acid linear peptide (PTH(1-84)) released from the parathyroid gland and is a peptide hormone that primarily activates the PTH1 receptor. PTH has been shown to be essential for PTH1 receptor activation, especially the N-terminal 34 residue (PTH(1-34), teriparatide), which has been utilized as a clinical therapeutic agent for osteoporosis. It is expected to develop a modified form of PTH with reduced side effects by controlling the activation time of the PTH1 receptor. ↑up
Note 2: Parathyroid hormone-related hormone (PTHrP )
PTHrP is a 141 amino acid linear peptide secreted from diverse cells including endocrine glands, blood vessels, bladder smooth muscle, uterine smooth muscle, mammary gland, epidermis, and central nervous system, and is a peptide hormone that selectively activates the PTH1 receptor. In particular, the N-terminal 36 residues (PTHrP(1-36)) have been shown to be essential for PTH1 receptor activation. Although this PTHrP (1-36) has a weaker osteogenic effect than PTH, its side effect of bone destruction is also reduced because it induces activation of a very short receptor. Therefore, it is hoped that clarification of the mechanism of this short receptor activation will provide specific guidelines for reducing the side effects of PTH ligands. ↑up
Note 3: Parathyroid hormone type 1 (PTH1) receptor
The PTH1 receptor is a G protein-coupled receptor that is activated by two endogenous hormones, PTH and PTHrP, and plays an essential role in bone formation and maintenance. Therefore, the development of drugs targeting the PTH1 receptor is expected to enable the treatment of a variety of diseases such as acute calciumemia, hypoparathyroidism, and osteoporosis. In particular, the activation of the PTH1 receptor by PTH administration significantly restores osteoporosis, which has led to the development of PTH- and PTHrP-modified osteoporosis drugs. ↑up
Note 4 G protein trimer
G protein trimer is a GTP-binding protein involved in intracellular signal transduction and is composed of a trimer of Gα, Gβ, and Gγ subunits, which are activated by agonist-bound GPCRs. The activated G protein dissociates into two subunits, Gα and Gβ-Gγ, with a GDP-GTP exchange reaction. Each dissociated subunit binds to a different downstream signaling factor and activates that signaling factor, resulting in various signaling responses in the cell. ↑up
Note 5: Single particle analysis method using cryo-electron microscopy
This method uses an electron microscope to capture images of a large number of biological macromolecules, including proteins, and reconstruct their three-dimensional structures by superimposing these images to determine the three-dimensional structure of the biological macromolecule. In this method, a sample cooled to a cryogenic temperature by liquid nitrogen (-196°C) is irradiated with electron beams, and the sample is observed by detecting the electron beams transmitted through the material. In the past decade, remarkable technological innovations have been made in sample measurement methods and detectors, and this technique has become widely known as a method for determining the three-dimensional structure of proteins at high resolution. For this technological innovation, the 2017 Nobel Prize in Chemistry was awarded to three overseas researchers who contributed to its development. ↑up
Note 6 G-protein-coupled receptor (GPCR)
GPCRs are 7-transmembrane proteins expressed at the plasma membrane and are known to form the largest family of proteins (about 800 species in humans) GPCRs are activated only by specific extracellular binding factors (ligands) and can activate intracellular G protein trimers by structural changes at the transmembrane site GPCRs are capable of activating intracellular G-protein trimers through conformational changes in the transmembrane domain. It is known that virtually all human biological phenomena are regulated by each receptor that has evolved to bind only specific ligands, and drugs targeting GPCRs account for more than 30% of all approved drugs. Class B1 GPCRs, the target of this study, consist of two domains, an extracellular domain and a transmembrane domain, and are the receptor group for peptide hormones. ↑
Note 7 Ligand
A ligand is a substance that binds specifically to a functional protein and changes the conformation of the target protein by binding to it. Ligands and target proteins are in a keyhole-key relationship, in which the ligand, which is the key, binds specifically to the keyhole of the target. ↑up
Note 8 Extracellular domain
This is a domain in the extracellular region consisting of 120-160 amino acids common to class B1 GPCRs. It is thought to function to accept long peptide hormones, which are ligands for class B1 GPCRs, and its detailed function differs depending on the receptor. ↑up
Note 9 Transmembrane domain
A domain consisting of seven transmembrane helices characteristic of GPCRs. Binding of an agonist causes the sixth transmembrane helix to undergo a major conformational change outward, allowing the binding of G protein trimers into the cell. ↑up


