DATE2022.07.22 #Press Releases
Elucidating the Origin of Interfacial Resistance in All-Solid-State Lithium Batteries
Disclaimer: machine translated by DeepL which may contain errors.
- Reducing Interfacial Resistance to 1/2,800, Contributing to Further Performance Improvements of All-Solid-State Batteries
Tokyo Institute of Technology
The University of Tokyo, Graduate School of Science
Summary
Project Associate Professor Kazuki Nishio and graduate student Daisuke Imaseki at the School of Materials Science and Engineering, Tokyo Institute of Technology, and Professor Taro Hitosugi at the Graduate School of Science, The University of Tokyo (concurrently serving as Project Professor of Applied Chemistry, School of Materials Science and Engineering, The University of Tokyo) have revealed that the high interface resistance between the solid sulfide electrolyte and electrode material in all solid-state lithium batteries is due to the formation of chemical reaction layers. The high interfacial resistance between the sulfide solid electrolyte and electrode material in all-lithium batteries is due to the formation of a chemical reaction layer. Furthermore, by introducing a buffer layer at the interface, they suppressed the formation of this chemical reaction layer and reduced the interfacial resistance to 1/2,800, demonstrating stable battery operation.
All-solid-state lithium batteries are expected to be used as large storage batteries for electric vehicles and stationary applications, and research and development is underway to achieve higher performance. In particular, there is an urgent need to reduce the high interfacial resistance between the solid sulfide electrolyte and electrode materials to achieve high-speed charging. However, the origin of this high interface resistance has been unknown.
In this study, it was found that when the Li3PS4 solid electrolyte and LiCoO2 electrode material contact each other to form an interface, structural changes and sulfur diffusion occur from the LiCoO2 electrode surface to a depth of about 10 nm. This is the chemical reaction layer, and its presence at the interface caused extremely high interface resistance and battery operation was not possible. However, when a buffer layer ( Li3PO4 thin film) was introduced to the interface, the formation of the chemical reaction layer was suppressed and an atomically ordered interface structure was maintained. This led to the realization of low interface resistance and stable charge-discharge. In addition, the interface between the sulfide solid electrolyte and electrode material was quantitatively evaluated for the first time.
This research is an important step toward further increasing the output of all-solid-state lithium batteries, which are expected to be put into practical use, by elucidating the origin of the interface resistance. The research results were published as an article in ACS Applied Materials and Interfaces, a journal of the American Chemical Society, on July 21 (U.S. time).
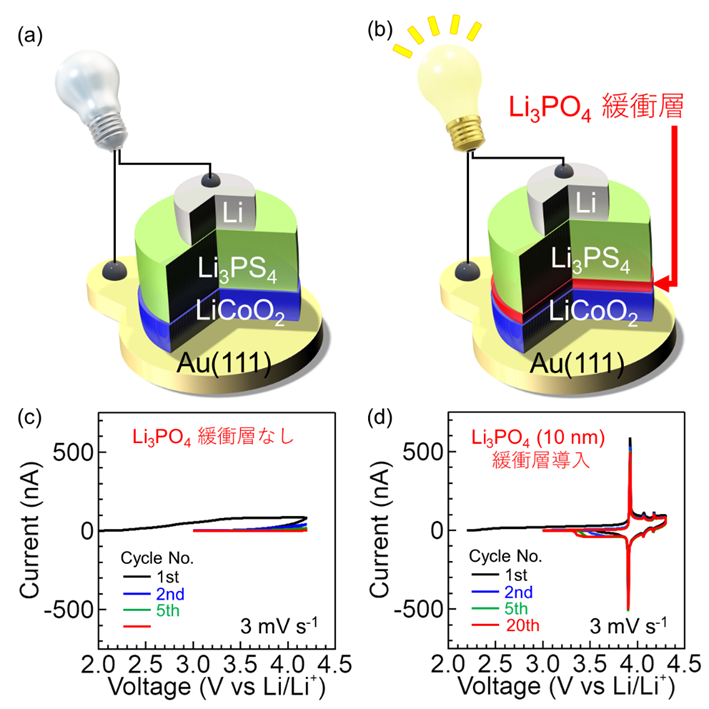
Figure: (a) Schematic of the fabricated thin-film all-solid-state Li battery. (b) Schematic diagram of the Li3PO4 oxide solid electrolyte introduced as a buffer layer at the interface between the Li3PS4 sulfide solid electrolyte and LiCoO2 electrode. (c) Cyclic voltammetry measurements without the Li3PO4 buffer layer. No sharp peaks were observed and no charge/discharge reaction occurred. (d) Measurement results with Li3PO4buffer layer (10 nm thick), showing charge and discharge reactions at 3.9 V vs. Li/Li+.
For more information, please refer to the Tokyo Institute of Technology website.


