DATE2022.01.26 #Press Releases
Achieved continuous synthesis of tamsulosin, the active pharmaceutical ingredient of Harnal, a treatment for dysuria.
Disclaimer: machine translated by DeepL which may contain errors.
Osamu Kobayashi, Professor, Department of Chemistry
Haruro Ishitani, Project Professor, Department of Chemistry
Yuki Saito, Project Assistant Professor, Department of Chemistry
Key points of the presentation
- The continuous synthesis of tamsulosin, the active pharmaceutical ingredient of the dysuria treatment (product name: Harnal) used for urinary tract stones and benign prostatic hyperplasia, has been achieved.
- Tamsulosin is approved and marketed in more than 90 countries worldwide as a treatment for dysuria, and demand for the drug is expected to increase further in an aging society.
- It has been demonstrated that the appropriate synthetic route design and the high-performance immobilized catalyst originally developed by the research group enable the consolidated and continuous production of optically active compounds with complex structures such as active pharmaceutical ingredients (Note 1).
Summary of the announcement
Continuous production of high value-added compounds such as pharmaceuticals is attracting attention as a next-generation manufacturing method that realizes a highly efficient, on-demand, and space-saving manufacturing process. While continuous synthesis has already been realized in the production of bulk chemicals such as petrochemicals, the development of continuous synthesis has been very limited in the synthesis of fine chemicals with complex structures, which require multi-step synthesis.
In this study, we have achieved the conjugated and sequential synthesis of tamsulosin, an active pharmaceutical ingredient, by utilizing the heterogeneous catalyst (Note 2 ) in a flow hydrogenation reaction. By designing a synthetic route based on catalytic hydrogenation and using an originally developed immobilized palladium catalyst, the hydrolysis reaction of the key phenethyl group and the reductive amination reaction with nitrile were realized in high yield under mild reaction conditions. This synthesis enabled the four-step reaction to obtain tamsulosin directly and successively in good yield without isolation and purification of intermediates.
The results of this research demonstrate that consolidated and continuous synthesis is possible by utilizing flow reactions that utilize high-performance heterogeneous catalysts. In the future, the development of heterogeneous catalysts with even higher activity and selectivity is expected to realize the continuous production of various pharmaceuticals.
The research results were published in the online bulletin of the German chemistry journal " Angewandte Chemie International Edition " at midnight on January 26, Japan time.
Publication details
Background of the Research
Tamsulosin(1a) is an α1-receptor blocker developed by Yamanouchi Pharmaceutical (currently Astellas Pharma Inc.), and is approved and marketed as a treatment for dysuria associated with benign prostatic hyperplasia (brand name: Harnal) in over 90 countries worldwide. It is the 28th most prescribed drug among all medicines in the U.S., and its demand is expected to increase further in the aging society. On the other hand, most compounds with complex structures such as pharmaceuticals are generally synthesized using a tank-type reactor called a batch method. The new method, called the continuous flow method (Note 3), is a manufacturing method in which raw material solutions are continuously pumped into a cylindrical reactor to continuously obtain the target product, and is more efficient, safe, and environmentally friendly than the conventional batch method. However, the flow synthesis of high-value-added compounds involves multi-step reactions without isolation and purification of intermediates, which is challenging because it requires minimizing the excess amount of reagents, by-products, and co-products in each reaction.
Description of Research
In this study, the consolidated and continuous synthesis of tamsulosin was achieved by designing a synthetic route based on hydrogenation and developing a highly functionalized immobilized palladium catalyst. The hydrogenation reaction using heterogeneous catalysts in the continuous-flow method proceeds efficiently because the gas and solid catalyst can react directly, unlike the reaction in the batch method. In addition, the co-products are harmless and easily removable compounds such as water, and the reaction is atomically efficient, making it a suitable reaction format for the consolidated and continuous flow methods. The research group has been developing original immobilized catalysts that greatly exceed the performance of commercial catalysts for flow hydrogenation reactions. In this synthesis, the immobilized palladium catalyst developed thus far is utilized, and by using appropriate reactants, all three steps of the asymmetric carbon building, deprotection, and skeleton formation reaction are performed in the hydrogenation reaction (Figure 1).
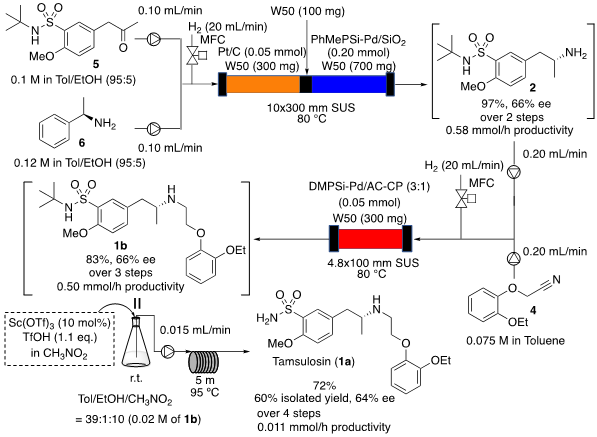
Figure 1: Linkage and sequential synthesis of (R)-tamsulosin
Using the easily synthesized ketone (5) as starting material, the first step is a stereoselective reductive amination reaction with optically active amine (6) using a Pt/C catalyst to afford the secondary amine (7) with good stereoselectivity.
In the second step, the key intermediate, optically active primary amine (2), is synthesized by the hydrolysis reaction of the phenethyl group. In this reaction, the originally developed immobilized palladium catalyst exhibits activity and durability far superior to those of commercially available palladium catalysts, and continuous reactions for 11 hours or longer were possible. The two-step reaction was also possible using a single column by packing two types of catalysts in the same column.
In the third step, the molecular skeleton of tamsulosin is formed by reductive C-N bond formation reaction. In the reductive C-N bond formation reaction, aldehydes and ketones are used as substrates, whereas nitriles (4), which are more stable, are used as substrates in this reaction. Although few C-N bond formation reactions using such substrates have been reported, we succeeded in obtaining the target product (1b) in high yield by using an immobilized palladium catalyst that we originally developed.
In particular, this reaction hardly progresses under batch conditions, whereas the target product was obtained under mild flow conditions, demonstrating the superiority of the flow reaction. The final step of deprotection of the tBu group catalyzed by Sc(OTf )3 was also carried out in the flow reaction, and the coupled and sequential synthesis of tamsulosin (1a) was achieved in a total yield of 60%. This synthesis enables direct and continuous synthesis of the final product without isolation and purification of intermediates, and the co-products are all volatile low molecular weight compounds such as water, ethylbenzene, ammonia, and isobutene, which can be easily separated from the product.
Future Developments
The results of this research demonstrate that catalytic hydrogenation and high-performance heterogeneous catalysts can be utilized for the conjugation and sequential synthesis of complex molecules. In particular, the continuous synthesis of optically active, high-value-added compounds such as pharmaceuticals is expected to be in increasing demand in the future, and the heterogeneous catalytic linkage synthesis demonstrated in this study is expected to be utilized to realize the continuous synthesis of a variety of pharmaceuticals.
This research was supported by the Drug Discovery Initiative of the Japan Agency for Medical Research and Development (AMED) and was conducted as part of the research project "Advanced Flow Precision Organic Synthesis for Real Production of Active Pharmaceutical Ingredients".
Journal
-
Journal name Angewandte Chemie International EditionTitle of paper Continuous-Flow Synthesis of (R)-Tamsulosin Utilizing Sequential Heterogeneous CatalysisAuthor(s) Yuki Saito, Ken Nishizawa, Benjamin Laroche, Haruro Ishitani, Shū Kobayashi *DOI Number 10.1002/anie.202115643
Terminology
1 Optically active compound
When a molecule cannot be superimposed on its own mirror image, it is called a chiral molecule, and pairs of mirror images are classified as "right-handed" and "left-handed". When either type is present in excess, the compound has optical rotation, and such compounds are called optically active compounds. Since most active pharmaceutical ingredients are chiral molecules and only one isomer has the desired bioactivity, very high optical purity is required for compounds. ↑up
Note 2: Heterogeneous catalysts
Catalysts are classified into homogeneous and heterogeneous catalysts. Homogeneous catalysts, such as metal complexes, are soluble in reaction solutions and are easy to synthesize and tune the structure. Heterogeneous catalysts are catalysts with active species immobilized on the solid itself or on the solid surface. In general, heterogeneous catalysts have the advantage that separation, recovery, and reuse of the catalyst can be easily achieved by filtration, while achieving high selectivity and leaching of the active species are problematic. ↑up
Note 3 Continuous flow method
The synthesis method in which reaction materials are continuously fed to the reactor and products are continuously removed from the reactor at the same time is called the continuous flow method. When heterogeneous catalysts are used, a cylindrical cartridge filled with catalyst is used as a reactor. By adjusting the pumping rate and operating time, this method can be used for synthesis of various scales, and has the advantages of space saving, high energy efficiency, and safety. ↑up


