DATE2021.12.09 #Press Releases
Platelets Predict Risk of Severe New Coronas
Disclaimer: machine translated by DeepL which may contain errors.
Keisuke Goda (Department of Chemistry, Professor / Adjunct Professor, University of California, Los Angeles / Adjunct Professor, Wuhan University)
Yutaka Yatomi (Vice President, Faculty of Medicine / Professor, Graduate School of Medicine)
Mako Nishikawa (Faculty of Medicine, Assistant Professor)
Hisashi Nitta (President, CYBO Co., Ltd.)
Key points of the presentation
- In novel coronavirus infection (COVID-19), thrombosis (especially microvascular thrombosis) has been shown to be one of the important factors in the severity and mortality of COVID-19. Indeed, autopsy (pathological autopsy) reports of patients who have died from COVID-19 have shown extensive microthrombosis within peripheral capillaries and microvessels of the lungs, heart, and other organs, which is assumed to be associated with multiorgan failure.
- Platelets play an important role in thrombus formation. In this study, to understand the process of microthrombus formation in COVID-19, blood samples from 110 COVID-19 patients admitted to the University of Tokyo Hospital were examined in detail, and surprisingly, an excessive number of circulating platelet aggregates The team discovered, for the first time in the world, that an excessive number of circulating platelet aggregates were present in about 90% of all patients. They also found a strong correlation between the frequency of platelet aggregates and the severity of illness, mortality, respiratory status, and vascular endothelial dysfunction in COVID-19 patients.
- The results of this study are expected to contribute to the elucidation of the pathogenesis of thrombosis in COVID-19, prediction of the risk of severe disease, exploration and evaluation of better antithrombotic therapy, and understanding of sequelae.
Summary of Presentation
Background of the study
In COVID-19, thrombosis (especially microvascular thrombosis) (Note 1)(Note 2) has been reported to be one of the important factors of severity and mortality in COVID-19, but its details have remained a mystery. To solve this mystery, a joint research group led by Professor Keisuke Goda of the University of Tokyo Graduate School of Science, Professor Yutaka Yatomi of the University of Tokyo Graduate School of Medicine, and Professor Gustavo Rohde of the University of Virginia, U.S.A., conducted a study of 110 COVID-19 patients admitted to the University of Tokyo Hospital (hereinafter referred to as "the University of Tokyo Hospital"). The group analyzed the big data of images of circulating platelet aggregates obtained from the University of Tokyo Hospital (Fig. 1, Fig. 2, and Fig. 3) by taking large-scale images of circulating platelet aggregates (Note 3) on a microfluidic chip using high-speed fluid imaging. As a result, surprisingly, we discovered for the first time in the world that an excessive number of circulating platelet aggregates exist in about 90% of all patients (Fig. 4). We also found a strong correlation between the frequency of circulating platelet aggregates and the severity of illness, mortality, respiratory status, and degree of vascular endothelial dysfunction in COVID-19 patients (Figures 4 and 5). The results of this research are expected to contribute to the elucidation of the mechanisms of thrombosis in COVID-19, prediction of the risk of severe disease, exploration and evaluation of better antithrombotic therapy, and understanding of the sequelae of the disease.
The research results were published online in Nature Communications on December 9, 2021 (7:00 PM).
Research Background
With the increasing number of COVID-19 case reports worldwide, it has become clear that thrombosis associated with COVID-19 is one of the important factors in the severity and mortality of COVID-19 COVID-19 causes a variety of thromboses in blood vessels throughout the body (cerebral infarction, myocardial infarction, deep vein thrombosis, pulmonary thromboembolism ) in blood vessels throughout the body. In addition, the risk factors for severe thrombosis in COVID-19, such as advanced age, underlying disease (diabetes, hypertension, malignancy, cerebrovascular disease, obesity, etc.), and smoking, are consistent with the risk factors for thrombosis. Indeed, autopsy reports of patients who died from COVID-19 have shown microvascular thrombosis, characterized by extensive microthrombi present in peripheral capillaries and microvessels in the lungs, heart, and other organs, suggesting an association with multiorgan failure. Recently, microthrombosis has been reported to be associated with sequelae after COVID-19 infection, and in response to numerous reports of improved prognosis with heparin-based anticoagulation in COVID-19 treatment, national and international medical institutions have begun to offer all COVID- 19 inpatients, clinical practice guidelines recommending thromboprophylaxis (primarily heparin therapy) have been published and its effectiveness is well understood. However, the pathogenesis of COVID-19-associated thrombosis remains unresolved and enigmatic.
Description of the Study
In this study, to understand the process of microthrombus formation in COVID-19, a collaborative research group led by Professor Keisuke Goda, Graduate School of Science, The University of Tokyo, Professor Hiroshi Yatomi, Graduate School of Medicine, The University of Tokyo, and Professor Gustavo Rohde, University of Virginia, USA, studied COVID- 19 patients (23 mild disease, 68 moderate disease, 19 severe disease; of which 73 males and 37 females; of which 99 survived and 11 died; total 110 patients) (Note 4) and examined platelet aggregates in blood samples in detail (Figure 1).
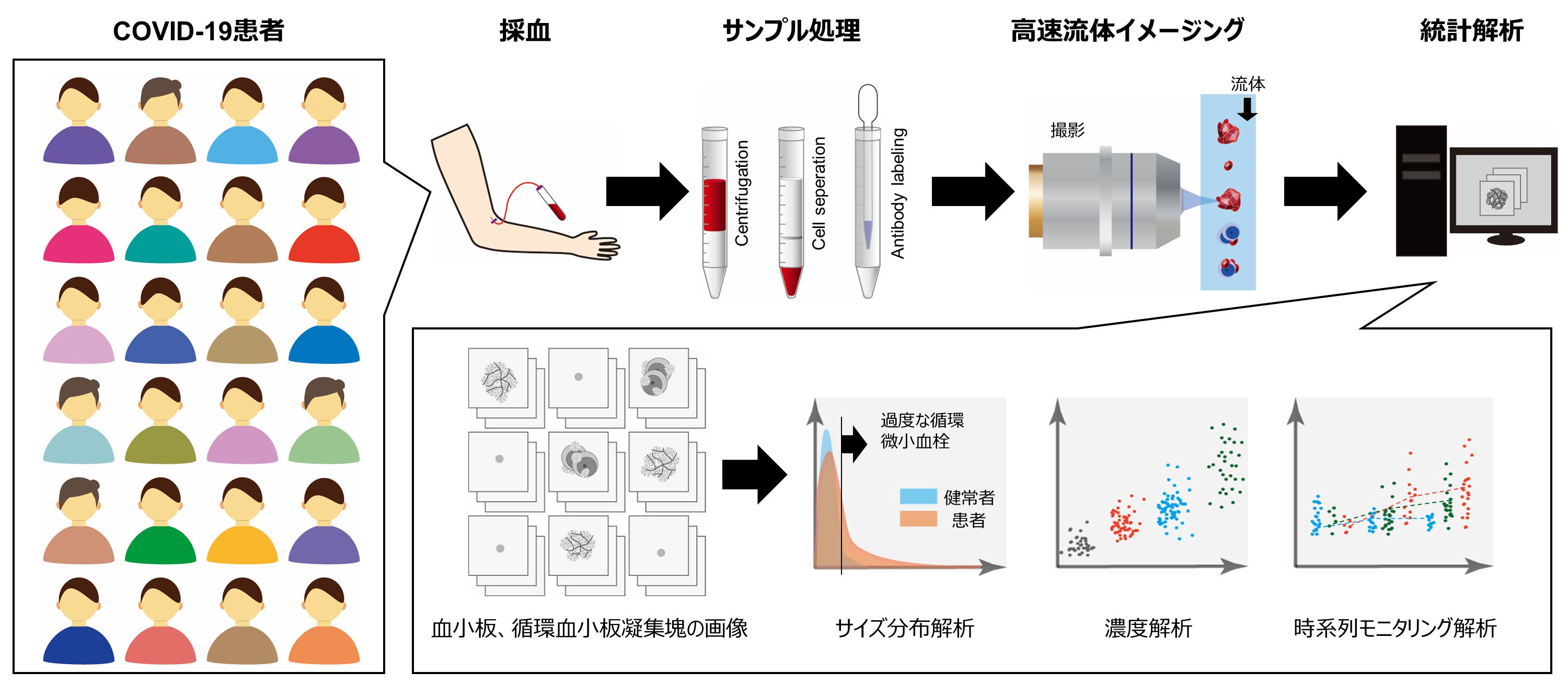
Figure 1: Conceptual diagram of the study. Blood samples from each patient were flowed on a microfluidic chip after processing, and many (25,000) images of platelets and circulating platelet aggregates per blood sample were obtained in a short time by high-speed fluid imaging technology to obtain image big data of circulating platelet aggregates and statistical analysis.
Specifically, blood samples of each patient were flowed on a microfluidic chip after processing three to five times a week, and a large number (25,000) of platelets and platelet aggregates (platelet-only aggregates and white blood cells) were obtained for each blood sample using a special high-speed fluid imaging technique installed in the Laboratory Division of the University of Tokyo Hospital in October 2020. The images of platelets and platelet aggregates (platelet-only aggregates, platelet aggregates containing leukocytes) were obtained in a short time using a special high-speed fluid imaging technique to obtain big data (Figure 2) of circulating platelet aggregates, and various statistical analyses were performed (Figure 3).
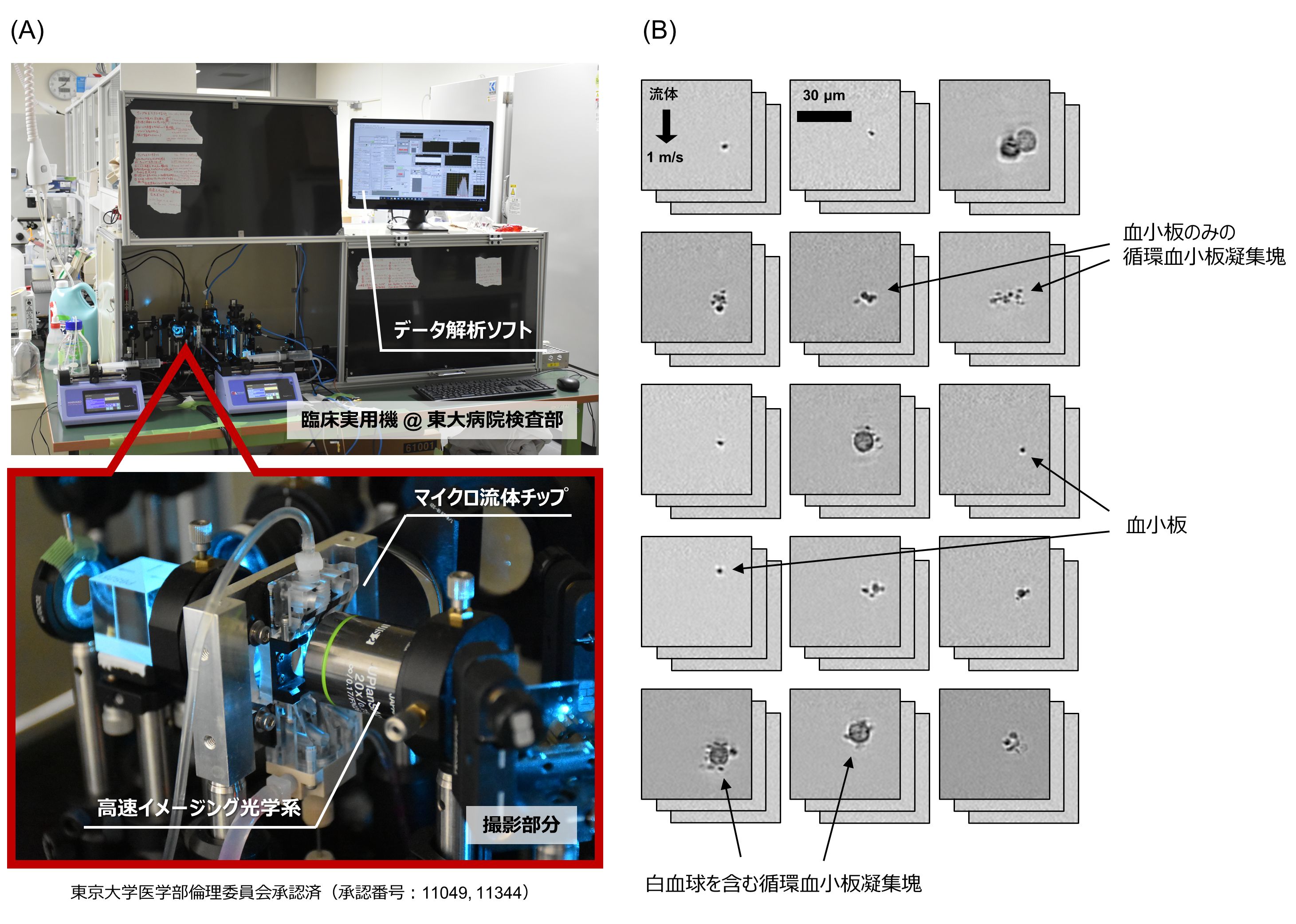
Figure 2: High-speed fluid imaging system and data analysis software used in this study. (A) Clinical practical machine installed in the laboratory of the University of Tokyo Hospital. (B) Acquired images of platelets and circulating platelet aggregates.
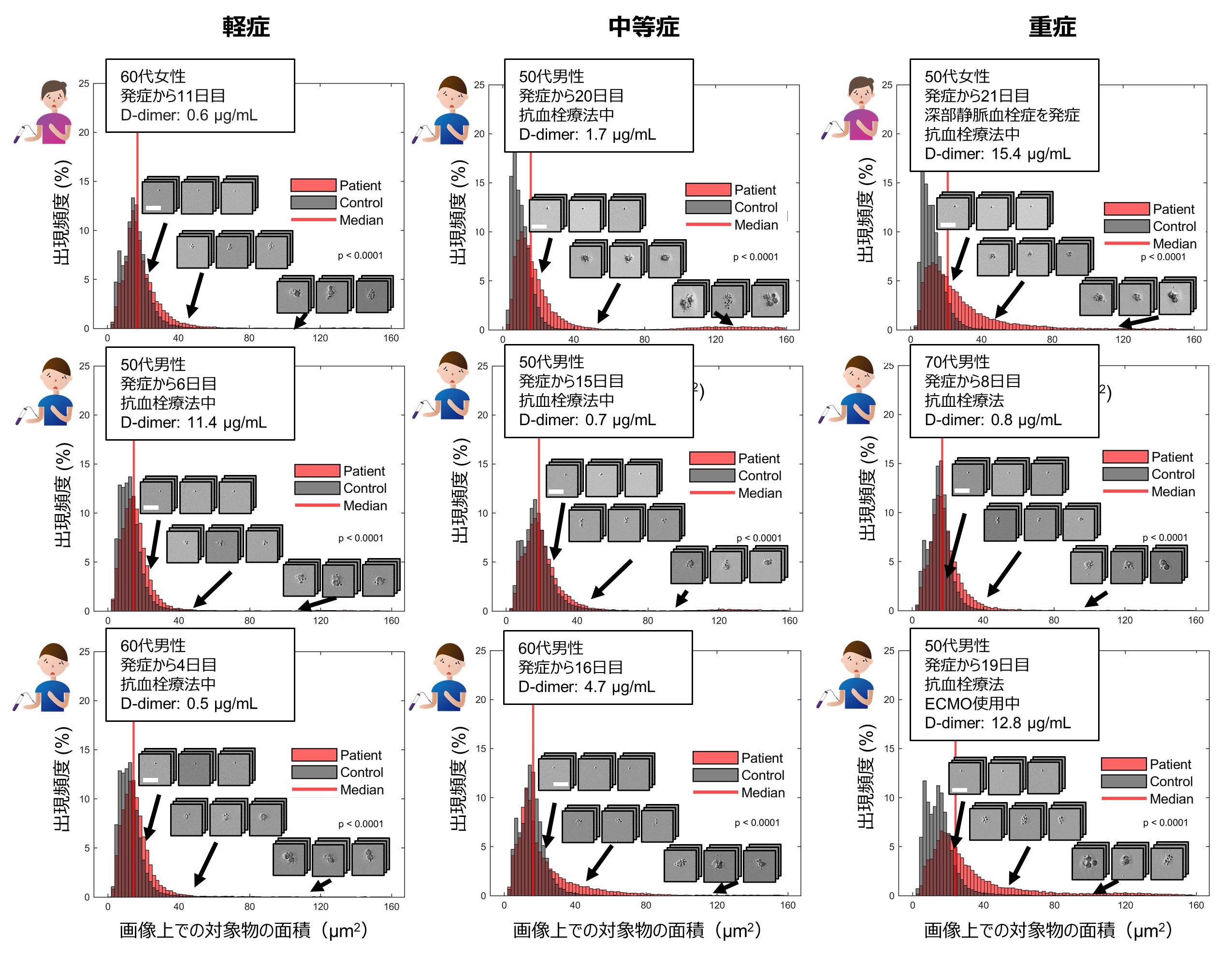
Figure 3: Size distribution of platelets and circulating platelet aggregates in typical mild, moderate, and severely ill patients.
Surprisingly, we discovered for the first time in the world that an excessive number of circulating platelet aggregates were present in 87.3% of all COVID-19 patients compared to healthy subjects. This included many patients whose D-dimer test (Note 5 ), which is widely used in screening tests for thrombosis, was below 1 µg/mL, the standard value at the University of Tokyo Hospital. We also found a strong correlation between the frequency of circulating platelet aggregates and the severity of illness and mortality of COVID-19 patients (Figures 4A and 4B). We also examined the effect of gender, and found that male patients had a higher frequency of platelet aggregates than female patients, although the statistical significance was not very high (Fig. 4C). This is consistent with previous reports that male patients are more likely to enter the intensive care unit and die than female patients. By performing image analysis, we also examined the structure of platelet aggregates and showed that the presence of leukocytes in circulating platelet aggregates is associated with severity and mortality in COVID-19 (Figure 4D). This is consistent with previous reports of increased leukocyte function in COVID-19 and the involvement of leukocytes in thrombosis.
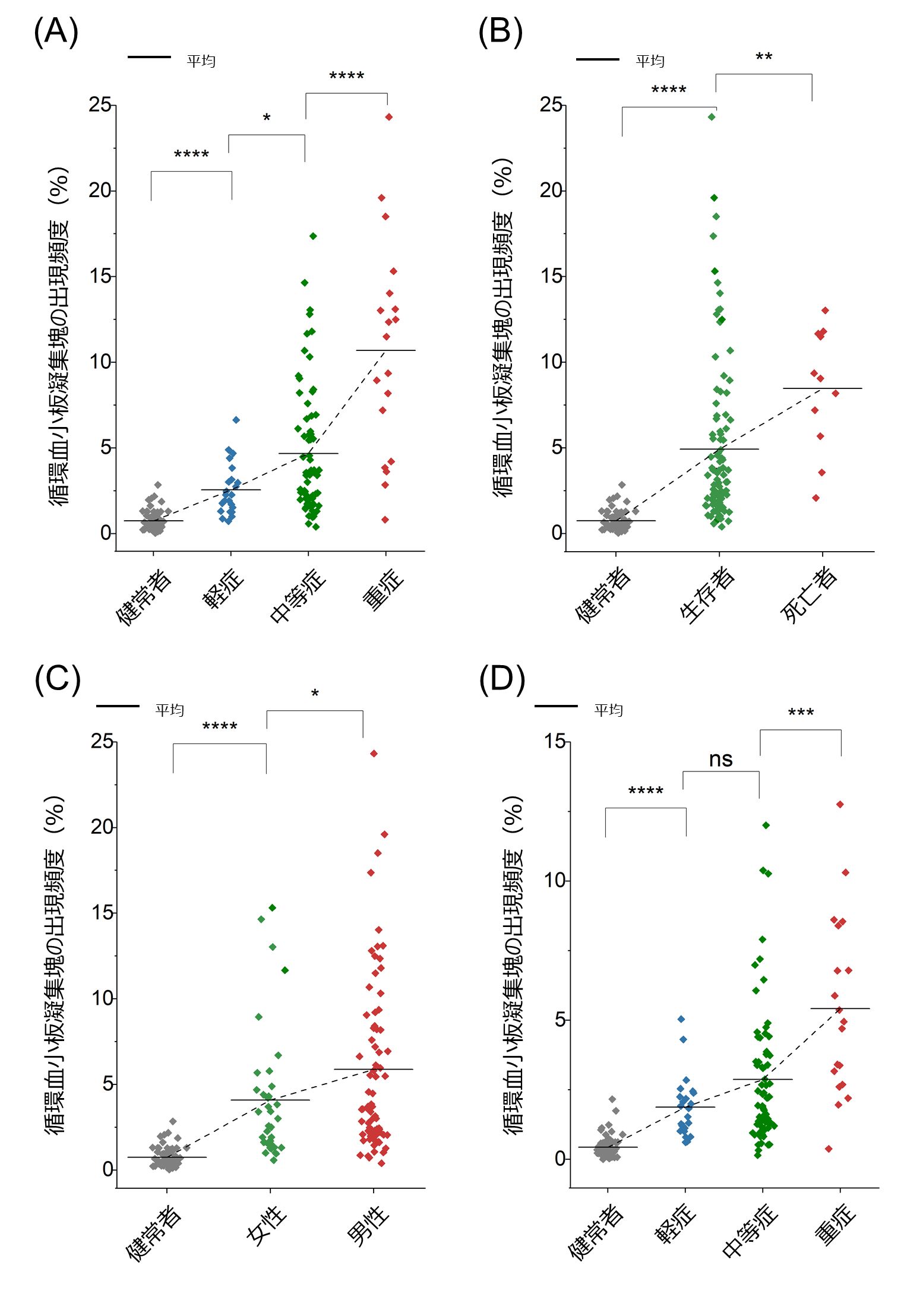
Figure 4: Statistical analysis of circulating platelet aggregates (ns: p > 0.05; *: p ≤ 0.05; **: p ≤ 0.01; ***: p ≤ 0.001; ****: p ≤ 0.0001). (A) Relationship between the frequency of circulating platelet aggregates and severity of disease. (B) Relationship between the frequency of circulating platelet aggregates and mortality. (C) Relationship between the frequency of circulating platelet aggregates and gender. (D) Relationship between the frequency of circulating platelet aggregates containing leukocytes and severity.
Next, we compared the frequency of appearance of circulating platelet aggregates with clinical laboratory data and found that the white blood cell count (WBC), D-dimer, coagulation factor VIII activity (FVIII), von Willebrand factor activity (VWF:RCo), thrombomodulin level (TM), and respiratory severity ( respiratory severity) were found to be strongly correlated with the frequency of circulating platelet aggregates (Figure 5).
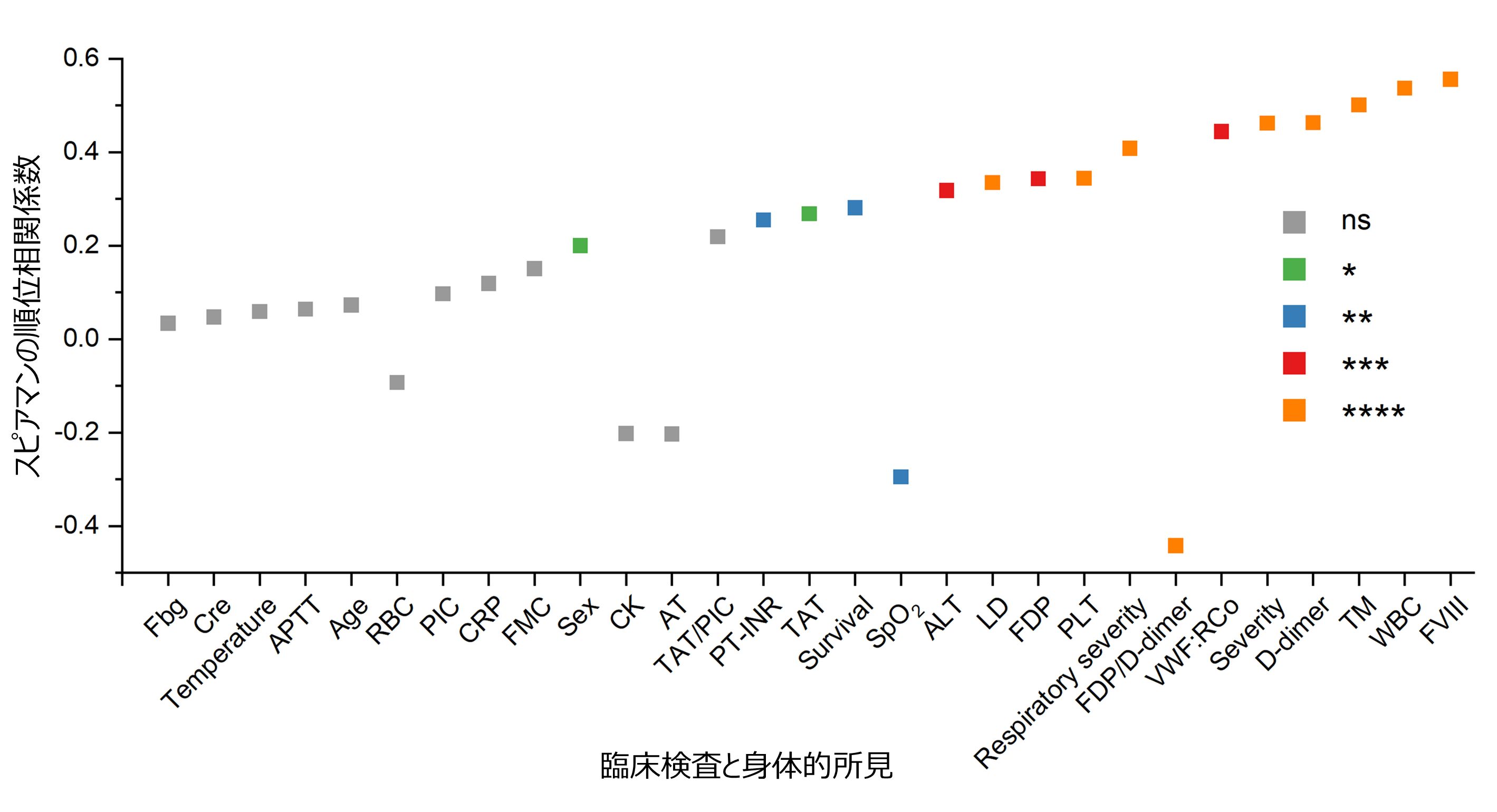
Figure 5: Relationship between frequency of appearance of circulating platelet aggregates and laboratory and physical findings (ns: p > 0.05; *: p ≤ 0.05; **: p ≤ 0.01; ***: p ≤ 0.001; ****: p ≤ 0.0001).
This means that circulating platelet aggregates are also associated with systemic thrombus formation, expressed in high D-dimer values, and vascular endothelial damage, expressed in high FVIII, VWF:RCo and TM values. These associations are consistent with previous reports of severe vascular endothelial damage in the lungs of COVID-19 patients and extensive microthrombus in alveolar capillaries. Furthermore, they appear to be consistent with reports that a novel coronavirus (SARS-CoV-2) can cause vascular inflammation following vascular endothelial damage, forming microthrombi and directly activating platelets.
Finally, the pathophysiology of COVID-19 patients by monitoring the frequency of circulating platelet aggregates over time has yielded interesting findings regarding prognosis (Figure 6A). Specifically, the frequency of circulating platelet aggregates in the mild disease group peaked on days 9-12 after onset, then gradually declined over the next week, and all mild disease patients were discharged on day 16. Similarly, the frequency of circulating platelet aggregates in the moderate disease group peaked on days 13 to 16 after onset and then decreased gradually over the next two weeks, with all moderate disease patients discharged on day 28. In contrast, the frequency of circulating platelet aggregates in the severe patient group peaked at 1 week after onset and then plateaued for 3 weeks, followed by death or transfer to a chronic care hospital. It is important to note that all patient groups have a moderate frequency of circulating platelet aggregates in the first 3-4 days after onset, but this begins to vary in the next 3-4 days, and while each patient group has a different prognostic pattern, the timing of discharge is consistent with a decrease in the frequency of circulating platelet aggregates in all prognostic patterns. This is the first time that a patient with thrombosis has been discharged from the hospital. It was also found that patients who developed thrombosis were hospitalized longer than patients with mild/moderate disease and some patients with severe disease. Furthermore, the strong correlation between respiratory status and the frequency of appearance of circulating platelet aggregates during the first two periods (days 1-9 and 10-18 of onset) indicates that circulating platelet aggregates are a good indicator of respiratory status in COVID-19 patients (Figure 6B). The bimodal distribution of the frequency of appearance of circulating platelet aggregates during the last period (after the 19th day after the onset of COVID-19) indicates that either patients at levels 2 and 3 (requiring oxygen administration) dropped to level 1 (no oxygen administration) and were discharged (recovered) or patients at level 4 (requiring ventilator or ECMO) required respiratory management and (requiring ventilator or ECMO) were discharged (recovered) or level 4 patients (requiring ventilator or ECMO) remained on respiratory management (remained hospitalized for a long time).
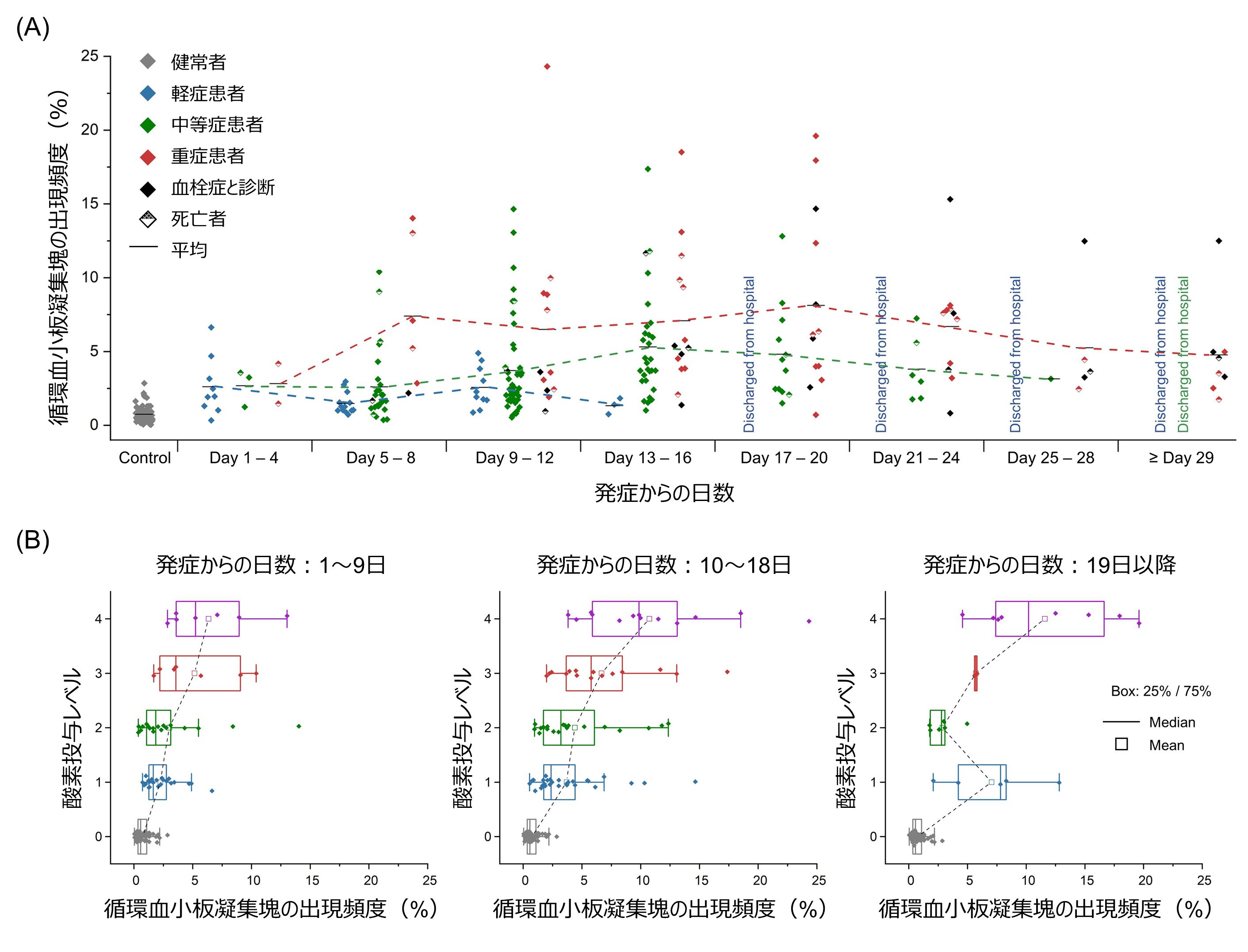
Figure 6: Time series monitoring of the frequency of circulating platelet aggregates. (A) Time series of the frequency of circulating platelet aggregates. (B) Relationship between the frequency of appearance of circulating platelet aggregates and the level of oxygen administration.
Future Developments
The findings of this study suggest that measuring the frequency and distribution of circulating platelet aggregates may be an effective approach to the diagnosis and treatment of COVID-19 in assessing the potential risk of microthrombus formation, which can only be confirmed by postmortem pathological autopsy. Indeed, there are numerous autopsy reports indicating that the primary cause of death in patients who died of pneumonia due to COVID-19 was respiratory failure due to diffuse alveolar damage with severe capillary stasis caused by microthrombi. The strong correlation between the frequency of circulating platelet aggregates and the severity of oxygen administration, as well as transcutaneous oxygen saturation ( SpO2 ) values, suggests that this technique can detect a wide range of precursors to microthrombus formation. The results of this study also suggest that COVID-19 patients who were not diagnosed with thrombosis may have formed microthrombi that were too small to be detected by medical imaging devices such as CT and MRI. Further studies are needed to directly verify the relationship between the frequency of circulating platelet aggregates and microvascular thrombosis. The University of Tokyo and CYBO Corporation signed a joint research agreement in February of this year, with the aim of commercializing the results of this research, among other things.
Research Team
The research team consists of Mako Nishikawa (Assistant Professor, Faculty of Medicine, The University of Tokyo), Hiroshi Kanno (Doctoral student, Graduate School of Science, The University of Tokyo), Yuqi Zhou (Doctoral student, Graduate School of Science, The University of Tokyo), Ting-Hui Xiao (Assistant Professor, Graduate School of Science, The University of Tokyo), Takuma Suzuki ( Graduate School of Frontier Sciences, The University of Tokyo), Yuma Ibayashi (Graduate School of Science, The University of Tokyo), Jeffrey Harmon (Graduate School of Science, The University of Tokyo), Shigekazu Takizawa (Graduate School of Science, The University of Tokyo), Kotaro Hiramatsu (Graduate School of Science, The University of Tokyo) Assistant Professor, Graduate School of Science, The University of Tokyo), Hisashi Nitta (President, CYBO Corporation), Risako Kameyama (Master's student, Graduate School of Science, The University of Tokyo), Walker Peterson (Doctoral student, Graduate School of Science, The University of Tokyo), Jun Takiguchi (Doctoral student, Graduate School of Medicine, The University of Tokyo), Mohammad Shifat-E-Rabii (Doctoral student, Department of Biomedical Engineering, College of Engineering, University of Virginia), Yan Zhuang (Doctoral student, Department of Electrical and Computer Engineering, College of Engineering, University of Virginia), Xuwang Yin (Doctoral student, Department of Electrical and Computer Engineering, College of Engineering, University of Virginia) Abu Hasnat Mohammad Rubaiyat (Doctoral student, Department of Electrical and Computer Engineering, College of Engineering, University of Virginia), Yunjie Deng (Master's student, Graduate School of Science, The University of Tokyo), Hongqian Zhang (Master's student, Graduate School of Science, The University of Tokyo), Shigeki Miyata (Vice Director, Central Blood Institute, Japanese Red Cross Society), Gustavo Rohde (Professor, Department of Biomedical Engineering, School of Engineering, University of Virginia), Wataru Iwasaki (Professor, Graduate School of Frontier Sciences, University of Tokyo), Hiroshi Yatomi (Professor, Graduate School of Frontier Sciences, University of Tokyo), Keisuke Goda (Professor, Graduate School of Medicine, University of Tokyo/) Keisuke Goda (Professor, Graduate School of Medicine, The University of Tokyo, Adjunct Professor, Department of Biomedical Engineering, Faculty of Engineering, University of California, Los Angeles, and Adjunct Professor, Wuhan University, School of Industrial Sciences).
Research Support
This work was supported by the Japan Agency for Medical Research and Development (AMED) under the Research Student Program for Emerging and Re-emerging Infections (JP20wm0325021), the Japan Society for the Promotion of Science (JSPS) under the Research Centers Initiative and Grant-in-Aid for Scientific Research (19H05633, 20H00317), the Cabinet Office's Program for Promoting Innovative R&D on Science and Technology (ImPACT The White Rock Foundation, Nakatani Foundation for Medical Instrumentation, Ogasawara Toshisho Memorial Foundation, Konica Minolta Science and Technology Foundation, Trust Fund for Promotion of Clinical Laboratory Medicine Research, National Institutes of Health (GM130825), National Science Foundation (1759802).
Journals
-
Journal name Nature Communications Title of paper Massive image-based single-cell profiling reveals high levels of circulating platelet aggregates in patients with COVID-19 Author(s) Masako Nishikawa, Hiroshi Kanno, Yuqi Zhou, Ting-Hui Xiao, Takuma Suzuki, Yuma Ibayashi, Jeffrey Harmon, Shigekazu Takizawa, Kotaro Hiramatsu, Nao Nitta, Risako Kameyama, Walker Peterson, Jun Takiguchi, Mohammad Shifat-E-Rabbi, Yan Zhuang, Xuwang Yin, Abu Hasnat Mohammad Rubaiyat, Yunjie Deng, Hongqian Zhang, Shigigeki Shigeki Hongqian Zhang, Shigeki Miyata, Gustavo K. Rohde, Wataru Iwasaki, Yutaka Yatomi, Keisuke Goda DOI Number 10.1038/s41467-021-27378-2
URL
Glossary of Terms
Note 1: Thrombosis
Thrombosis is a disease in which a blood clot (thrombus) formed in the blood from various causes occludes a blood vessel and causes organ damage due to peripheral circulatory failure, or a formed thrombus is carried away by the blood flow and occludes a blood vessel in a different site than the site of formation, causing organ damage. It is classified into arterial thrombosis (cerebral infarction, myocardial infarction, peripheral arterial thrombosis, etc.) and venous thrombosis (deep vein thrombosis, pulmonary thromboembolism, etc.). ↑up
Note 2 Microvascular thrombosis
Among thrombosis, this refers to thrombosis occurring in microvessels. ↑↑
Note 3 Circulating platelet aggregates
A platelet aggregate (platelet-only aggregate, platelet aggregate containing leukocytes, etc.) formed when platelets are activated by some stimulus. It is suggested that they circulate in the body before and after microthrombus formation in this study. ↑up
Note 4 Severity of COVID-19 patients
The severity of illness in this study was in accordance with the severity criteria established by the Tokyo Metropolitan Government. Mild disease: Level not requiring oxygen administration. Moderate disease: Level requiring oxygen administration. Severe: Level requiring ventilatory management or artificial lung ECMO. ↑up
Note 5 D-dimer test
A test that measures the breakdown product of stabilized fibrin, which is broken down and produced by thrombin, a product of the coagulation cascade. It is primarily used clinically in the evaluation of patients with suspected venous thromboembolism (deep vein thrombosis, pulmonary embolism, etc.). ↑up


