DATE2021.03.31 #Press Releases
Asgard Archaea's ability to use light energy to
structure of proteins that take up hydrogen ions.
Disclaimer: machine translated by DeepL which may contain errors.
Masamitsu Higuchi (2nd year, Master's student, Department of Biological Sciences, at the time of research)
Wataru Shihoya (Assistant Professor, Department of Biological Sciences)
Masae Konno (Project Researcher, Institute for Solid State Physics, The University of Tokyo)
Hideki KAMITORI (Professor, Graduate School of Engineering, Nagoya Institute of Technology)
Keiichi Inoue (Associate Professor, Institute for Solid State Physics, The University of Tokyo)
Osamu Nureki (Professor, Department of Biological Sciences)
Key points of the presentation
- The steric structure of sizorhodopsin, a type of rhodopsin protein (Note 1) possessed by Asgard Archaea, which functions to take hydrogen ions into the cell using light energy.
- The structure of schizorhodopsin has a short portion facing the intracellular side of the protein, and the mechanism by which it efficiently transports hydrogen ions into the cell has been elucidated.
- This study demonstrates that schizorhodopsin has evolved to efficiently transport hydrogen ions into the cell and, based on the structural information, paves the way for the modification and functionalization of schizorhodopsin and its application as a molecular tool for the study of cranial nerve diseases and acidosis (Note 2 ) and other diseases.
Summary of the presentation
All eukaryotes are thought to have evolved 1.5 to 2.0 billion years ago from organisms called archaea, which do not have nuclei. Among existing organisms, the Asgardian Archaea is the closest to the Archaea from which eukaryotes originated. In 2020, a light-responsive rhodopsin, schizorhodopsin, was discovered in Asgard Archaea, and its ability to transport hydrogen ions into the cell was first reported. It is possible that the process of transformation of Asgard Archaea into eukaryotes involves the uptake of hydrogen ions by schizorhodopsin in order to adapt to an environment with sunlight and oxygen. However, the mechanism of how schizorhodopsin efficiently transports hydrogen ions into the cell was unknown.
In this study, Professor Nureki's group at the Graduate School of Science, The University of Tokyo, in collaboration with Associate Professor Inoue at the Institute for Solid State Physics, has determined the three-dimensional structure of sisorhodopsin by X-ray crystallography (Note 3). By comparing the structure of sizorhodopsin with other rhodopsins, the position of sizorhodopsin in the molecular evolution of rhodopsin, which was previously unknown, was clarified. In addition, the short transmembrane region on the intracellular side of schizorhodopsin facilitates the release of hydrogen ions to the intracellular side of the protein, revealing a hydrogen ion transport mechanism different from that of known rhodopsins, in which hydrogen ions taken in from outside the cell are released directly into the solvent on the intracellular side of the cell. This research is expected to make it possible to modify the function of schizorhodopsin based on structural information and to advance research on its application as a molecular tool for medical research.
The research results were published in Proceedings of the National Academy of Sciences of the United States of America (PNAS) on March 3031, 2021 Japan time.
Publication details
Background of the research
Animals and plants, including humans, are called eukaryotes because their cells contain a nucleus that encases DNA encoding genetic information. On the other hand, primitive organisms such as Escherichia coli do not have a nucleus in their cells and are called prokaryotes. In 2020, a research group led by the Japan Agency for Marine-Earth Science and Technology (JAMSTEC) reported a study of Asgardian Archaea culture (Imachi et al. (Imachi et al, 2020) (Fig. 1, left), and the boundary between prokaryotes and eukaryotes is being actively studied.
The Asgard Archaea genome information contained an unprecedented type of rhodopsin that uses light energy to perform a variety of physiological functions. This is called schizorhodopsin, a molecule that functions to use light energy to bring hydrogen ions into the cell (Fig. 1, right) (Inoue et al. 2020). During the transformation of Asgard Archaea into eukaryotes, the uptake of hydrogen ions by schizorhodopsin may be involved in their adaptation to an environment with sunlight and oxygen. Proteins from another type of organism found in Antarctica have a similar sequence and function, and schizorhodopsin is widely distributed in the biological world.
The amino acid sequence of schizorhodopsin is somewhere between the long-known type 1 group represented by bacteriorhodopsin (Note 4 ) and a new species found in 2018, heliorhodopsin (Note 5), and we did not know which group it would fall into (Figure 1 right).
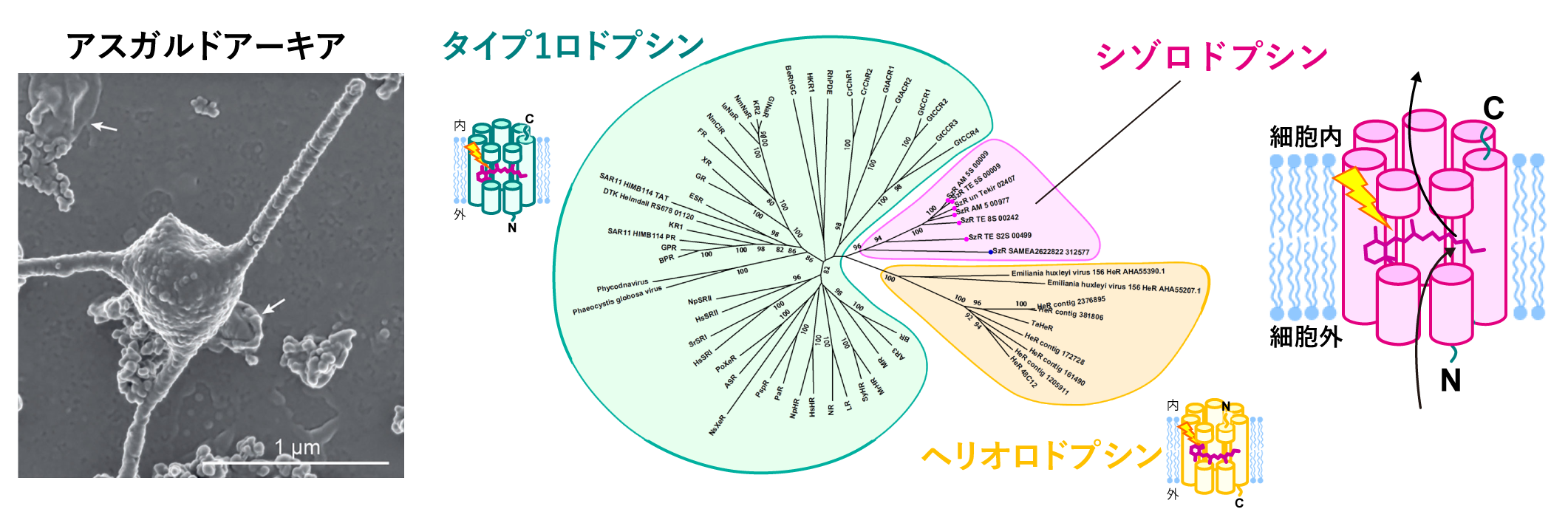
Figure 1: Introduction of schizorhodopsin.
(Left) Photograph of a culture-isolated Asgardian Archaea closely related to those with schizorhodopsin ((English) H. Imachi, M. K. Nobu, and JAMSTEC; (Japanese) Hiroyuki Imachi, Yu Nobu, and JAMSTEC). It has long tentacle-like projections.
(Right) Phylogenetic tree of rhodopsin discovered so far and schematic diagram of schizorhodopsin.
The large number of rhodopsins that carry hydrogen ions in microorganisms are mostly those that carry them outside the cell, such as bacteriorhodopsins, and only a few carry them inside the cell. Among these rhodopsins, schizorhodopsin has been shown to be a very efficient transporter compared to other inward-transporting rhodopsins. The structure of schizorhodopsin, which differs significantly from these rhodopsins in its amino acid sequence, and the mechanism by which it transports hydrogen ions into the cell were not known.
Research Details and Results
The research group clarified the three-dimensional structure of schizorhodopsin using X-ray crystallography (Figure 2).
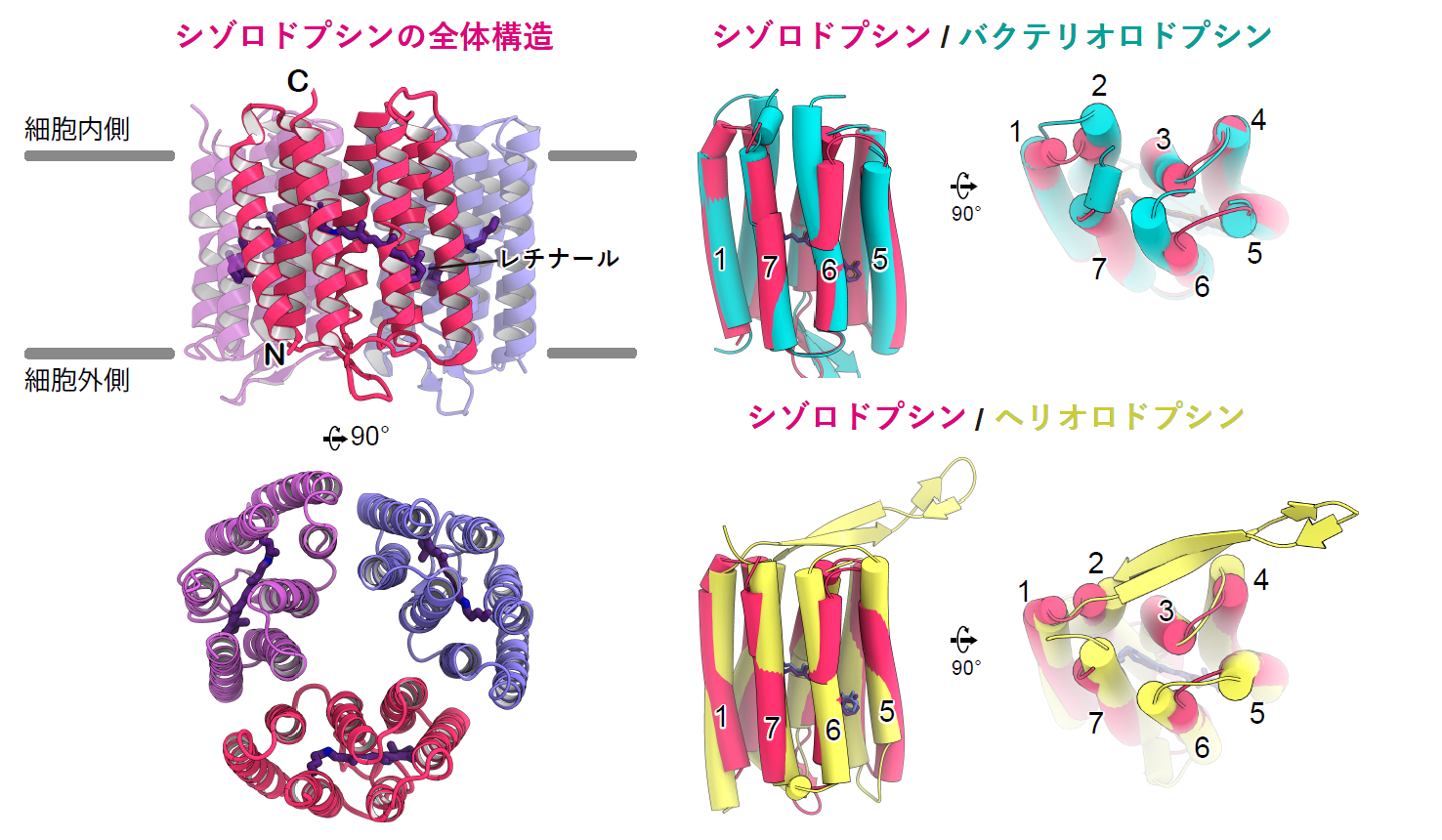
Figure 2: Structure of schizorhodopsin
(Left) Overall structure of the sizorhodopsin trimer
(Right) Superposition of sizorhodopsin with bacteriorhodopsin and heliorhodopsin.
Schizorhodopsin formed a trimer, which, like rhodopsin up to now, was composed of seven transmembrane helices (TM) and a retinal dye (Note 6) shared with lysine, one of the amino acids. To investigate whether schizorhodopsin is more similar to type 1 rhodopsin or heliorhodopsin, we compared the structures and found that the intracellular and extracellular loop structures of schizorhodopsin are close to those of bacteriorhodopsin and very different from those of heliorhodopsin. Thus, we found that schizorhodopsin can be classified as an existing type 1 protein, although the sequence of amino acids constituting the protein is intermediate between the two.
Next, the transport mechanism of hydrogen ions was analyzed. In the dark state, hydrogen ions reside in the Schiff base of the retinal, and upon light stimulation, they leave there and are released into the cell through the glutamate present on the intracellular side of schizorhodopsin (Figure 3 left). In a typical hydrogen ion pump, hydrogen ions are transported by ballistic transfer between amino acid residues such as glutamate and water molecules. However, in schizorhodopsin, although this glutamate is essential for the transport of hydrogen ions into the cell, hydrogen ions are not trapped in this amino acid, suggesting that hydrogen ions are released directly from the protein into the cell, and the hydrogen ion transport mechanism of schizorhodopsin is a great mystery The mechanism of hydrogen ion transport of schizorhodopsin has remained a major mystery. Characteristically, the intracellular-facing portion of sizorhodopsin was shorter than that of other rhodopsins, especially TM6, which was about 13 amino acid residues shorter (Figure 2). These structural features indicate that the glutamate on the intracellular side is easily exposed to the solvent (Fig. 3, left). Experiments using the amino acid modified protein (Note 7) in the path of hydrogen ions also showed that solvent easily flows into the interior of the protein (Figure 3 right).
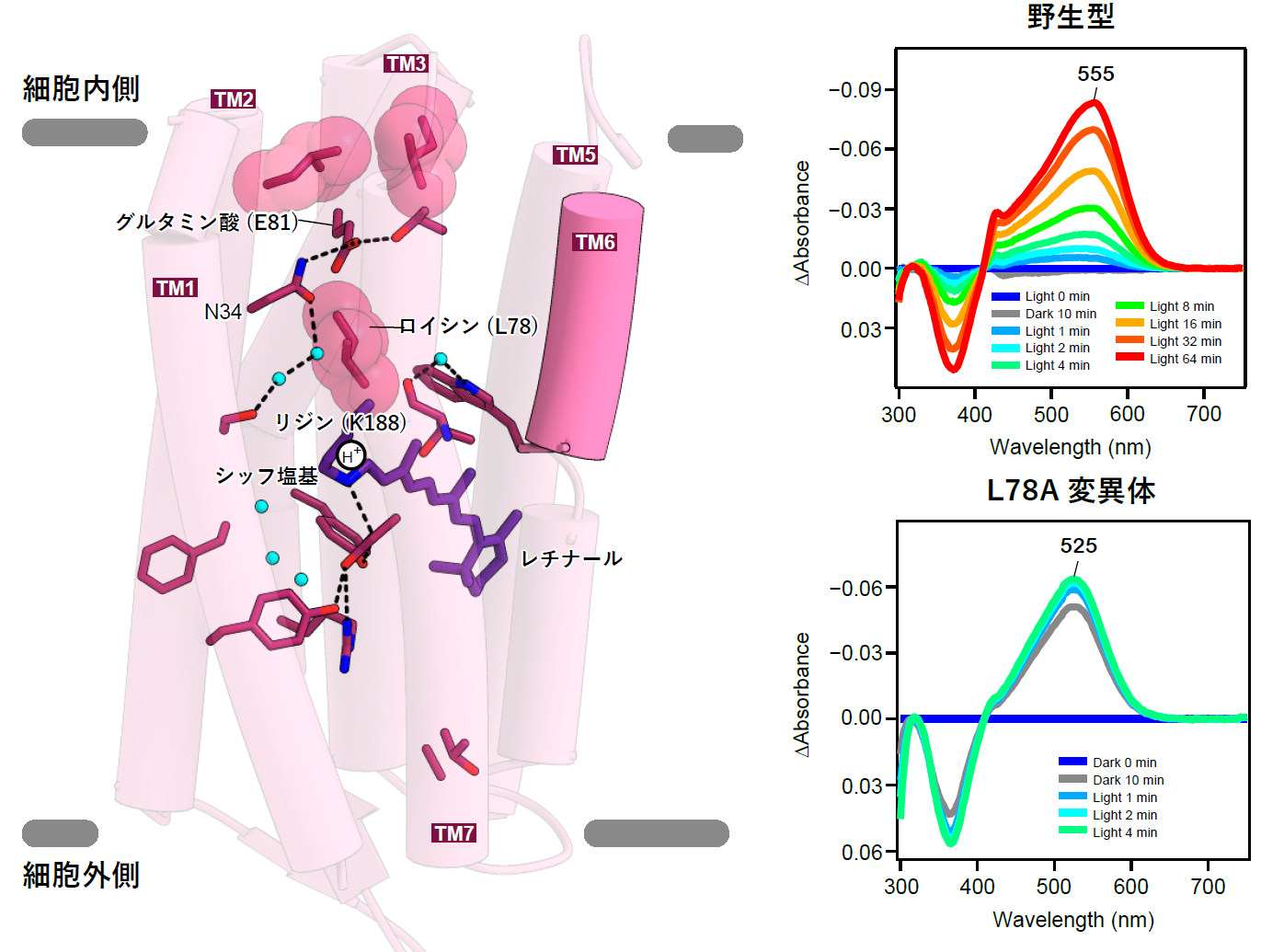
Figure 3: Characteristics of schizorhodopsin, whose intracellular side is easily exposed to solvent.
(left) Hydrogen ion transport pathway of schizorhodopsin focusing on the intracellular side. Because TM6 is short, it cannot cover the inside of the cell like other rhodopsins, and glutamic acid 81 (E81) is separated from the solvent only by two leucines. Between the Schiff base and E81, leucine 78 blocks the pathway, and during photoactivation, the movement of leucine 78 is thought to be responsible for the movement of hydrogen ions.
(Right) Change in light absorption of the protein showing that in the mutant with altered leucine 78 (bottom), water and reagents from outside enter even inside the protein, and retinal react more readily even without light than in wild-type schizorhodopsin (top).
Based on these results, we proposed a unique inward proton transport model for schizorhodopsin in which light-induced conformational changes allow solvent to enter the protein and hydrogen ions to be released directly into the cell, induced by glutamate (Figure 4).
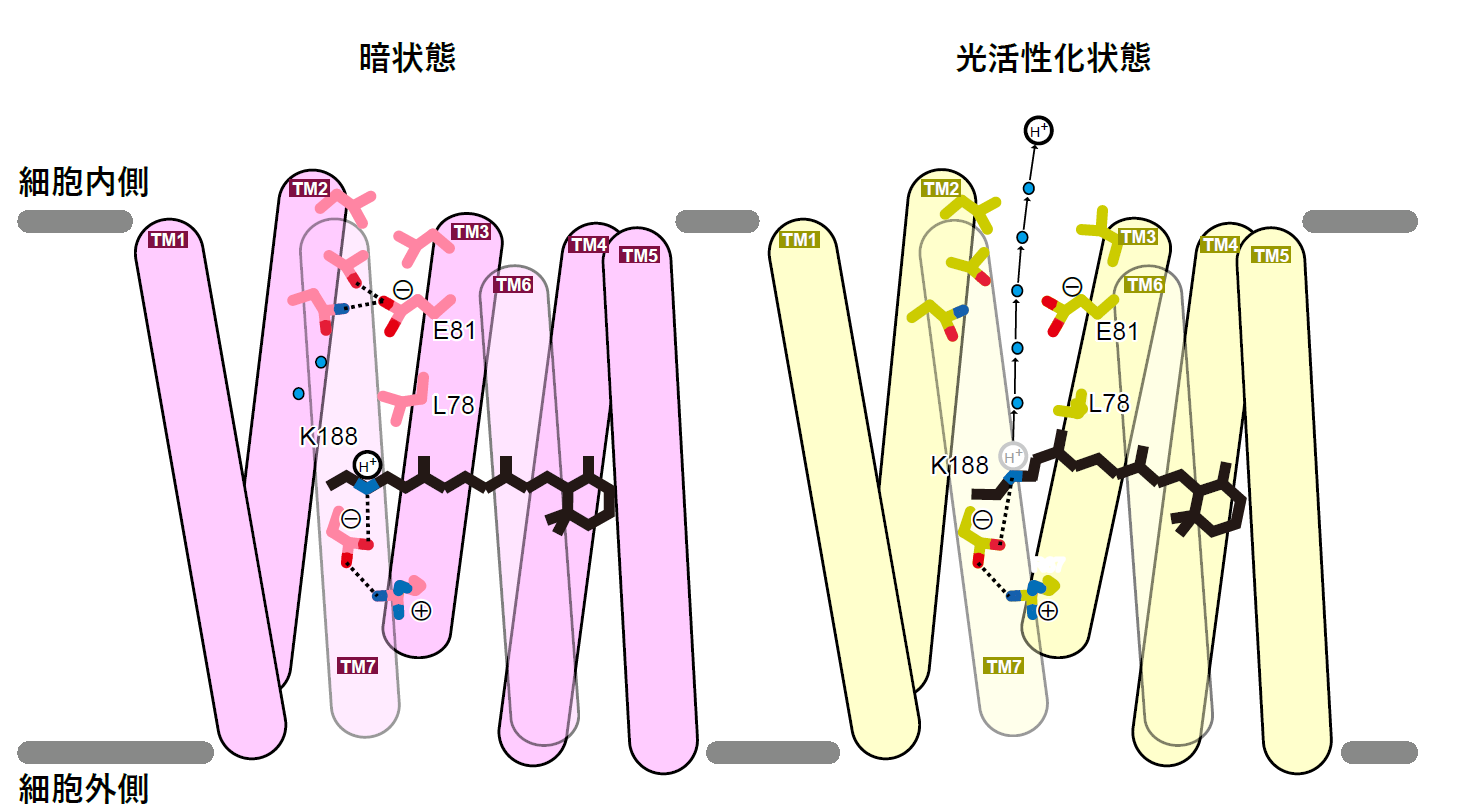
Figure 4: The inward hydrogen ion transport model of schizorhodopsin.
This research was supported by Grant-in-Aid for Specially Promoted Research on Innovative Areas, "Elucidation of Molecular Mechanisms of Membrane Proteins Regulated by Physical Stimuli" (Project Leader: Osamu Nureki) and by Grant-in-Aid for Scientific Research (B) "Elucidation of Transport Mechanism of Light-Driven Inward Proton Pump and Its Application to Optogenetics (Project Leader: Keiichi Inoue) and Young Scientists' Research "Structure-function analysis of non-classical rhodopsin" (Project Leader: Wataru Shihoya, 20K15728). This research was also conducted as part of the "Drug Discovery Initiative" of the Japan Agency for Medical Research and Development (AMED), which aims to link the results of outstanding life science research to the practical application of pharmaceuticals and other products by opening up large facilities such as synchrotron radiation facilities to the outside world. The project was supported by the "Platform for Advanced Technology Support for Drug Discovery (BINDS). This project was also supported by the Japan Science and Technology Agency (JST) CREST "Development and Application of Optical Manipulation of Intracellular Secondary Messengers" (Principal Investigator: Hideki Kandori).
Future Prospects
This study indicates that the form of schizorhodopsin has evolved for efficient uptake of hydrogen ions into the cell, suggesting its physiological importance in Asgardian archea. Future studies will be needed to elucidate the role of cellular inward hydrogen ion transport in the original Asgard Archaea. The mechanism revealed in this study, which releases hydrogen ions directly into the cell, is a breakthrough in the mechanism of hydrogen ion transport in vivo and is expected to lead to a better understanding of the molecular mechanisms of other proteins. The expression of schizorhodopsin in neurons is expected to make it possible to manipulate the excitation of any neuron by light, and will be used as a molecular tool for research on the pathogenic mechanism of depression and other disorders related to neurons in the brain, and for clarifying the mechanism of acidosis, a cellular disease associated with acidification of the blood. It is also expected to be used as a molecular tool to elucidate the mechanism of acidosis, a cellular disease associated with acidification of the blood.
Journals
-
Journal name Proceedings of the National Academy of Sciences of the United States of America Title of paper Crystal structure of schizorhodopsin reveals mechanism of inward proton pumping Author(s) Akimitsu Higuchi‡, Wataru Shihoya‡*, Masae Konno, Tatsuya Ikuta, Hideki Kandori, Keiichi Inoue*, Osamu Nureki* DOI Number URL https://doi.org/10.1073/pnas.2016328118
Terminology
Note 1 Rhodopsin protein.
A protein that absorbs sunlight and expresses various biological functions on the cell membrane of a wide range of organisms, from animals to bacteria and other microorganisms. Animal rhodopsins are receptors for light-related signal transduction such as vision, whereas most microbial rhodopsins use light energy to transport ions. Thus, although their functions are very different from each other and they are evolutionarily completely different lineages, they share many similarities, such as the retinal pigment, a derivative of vitamin A, bound inside the protein to absorb light, and the protein structure consisting of seven helixes that pass through the membrane. ↑up
Note 2 Acidosis
Acidosis is a disease in which the human blood is acidified for some reason, although it is normally maintained at a neutral level. Acidosis causes a large amount of hydrogen ions in the blood, which may damage tissues and cells. ↑up
Note 3 X-ray crystallography
An experimental technique to elucidate the three-dimensional structure of molecules by analyzing diffraction images obtained by irradiating X-rays to crystals formed by regularly arranged molecules. Along with cryo-electron microscopy and NMR, it is used to analyze the three-dimensional structure of proteins. ↑up
Note 4 Bacteriorhodopsin
The first microbial rhodopsin discovered, which acts as a light-driven hydrogen ion efflux pump. By creating a concentration gradient of hydrogen ions inside and outside the cell, this is used by another protein to produce energy. ↑up
Note 5: Heliorhodopsin.
A membrane protein that covalently binds to the cofactor retinal and photoreceptors. It can be divided into two major types: microbial rhodopsin and animal rhodopsin. ↑up
Note 6 Retinal pigment
Amino acids normally do not absorb light in the visible region, and proteins composed of amino acids cannot use visible light by themselves. Rhodopsin, on the other hand, has a pigment called retinal, which is produced from vitamin A in an enzymatic reaction in the body, bound inside the protein. When the retinal inside the rhodopsin protein absorbs visible light, its structure changes, which in turn causes changes in the protein portion, enabling it to express various physiological functions. ↑up
Note 7: Amino acid modified proteins
The rhodopsin protein is composed of approximately 200 amino acids, but by using genetic engineering techniques, amino acids located anywhere on the protein can be replaced with another type of amino acid (there are generally 20 types of amino acids in the body of organisms). The amino acid-modified proteins are produced in this way, and their functions and physical properties can be examined to determine the role of each amino acid in the main body protein. ↑up


