DATE2021.02.19 #Press Releases
Discovery of a new ice phase (ice XIX) at low temperatures and high pressure
Disclaimer: machine translated by DeepL which may contain errors.
Yoshiaki Yamane, Project Researcher, Institute for Solid State Physics (3rd year of Doctoral Program, Department of Chemistry)
Issei Komatsu, Associate Professor, Geochemical Research Center
Jun Goji, Assistant Professor, Institute for Solid State Physics
Miya Kamitoko, Professor, Institute for Solid State Physics
Shinichi Machida (Researcher, Institute of Engineering Innovation)
Takanori Hattori (Senior Staff, Japan Atomic Energy Agency)
S. Ito (Department of Chemistry, 1st year, Master course)
Hiroyuki Kagi, Professor, Geochemical Research Center
Key points of the presentation
- We discovered a new polymorph of ice (ice XIX) by using our originally developed dielectric constant measurement system under low temperature and high pressure (Note 1) and neutron diffraction experiments (Note 2).
- This ice XIX is a hydrogen-ordered phase of ice VI, but it is a different ordered phase from the previously known hydrogen-ordered phase of ice VI (ice XV), and it is the first demonstration of the existence of multiple ordered phases corresponding to a single hydrogen-disordered phase.
- The temperature-pressure phase diagram of ice VI and the two ordered phases is clarified, and it is shown that the two different ordering is realized by the difference in pressure.
Summary of Presentation
Polymorphs are those having the same chemical composition but different crystal structures, such as graphite and diamond. Polymorphs also exist in ice, but the variety of polymorphs is outstanding compared to other substances. In this study, we discovered a new polymorph of ice, the 20th (Note 3 ). It is astonishing that ice consisting only of H2O molecules has 20 different crystal structures. Most of the polymorphs of ice appear at very high pressures of the order of GPa (Note 4). Since the early 1900s, many researchers have developed high-pressure experimental techniques to generate pressures above 1 GPa, and as these techniques developed, new polymorphs of ice were discovered one after another. For example, Percy Bridgman, the father of high-pressure physics and winner of the Nobel Prize in Physics in 1946, discovered the four phases of ice IV, V, VI, and VII (the Roman numbers attached to each polymorph indicate the order in which they were discovered; normal ice produced in a freezer is called ice Ih(Note 3 )). Most recently, ice XVIII was reported in 2019 under extreme conditions, such as above 100 GPa and 2000 K.
In collaboration with the Institute for Solid State Physics, the Institute of Neutron Science of the Cryogenic Research Center and the J-PARC Center of the Japan Atomic Energy Agency, a research group led by Dr. Katsuya Yamane, Research Fellow at the Institute for Solid State Physics, Associate Professor Issey Komatsu and Professor Hiroyuki Kagi of the Graduate School of Science of the University of Tokyo, has now obtained new dielectric constants at low temperatures and high pressure, and measured the dielectric constant of ice XVIII at high pressure. The research group has discovered a new polymorph of ice, ice XIX, through dielectric measurements and neutron diffraction experiments at low temperatures and high pressures. This ice is obtained by pressurizing water at room temperature and cooling the first appearance of ice VI to about -150 °C (Figure 1).
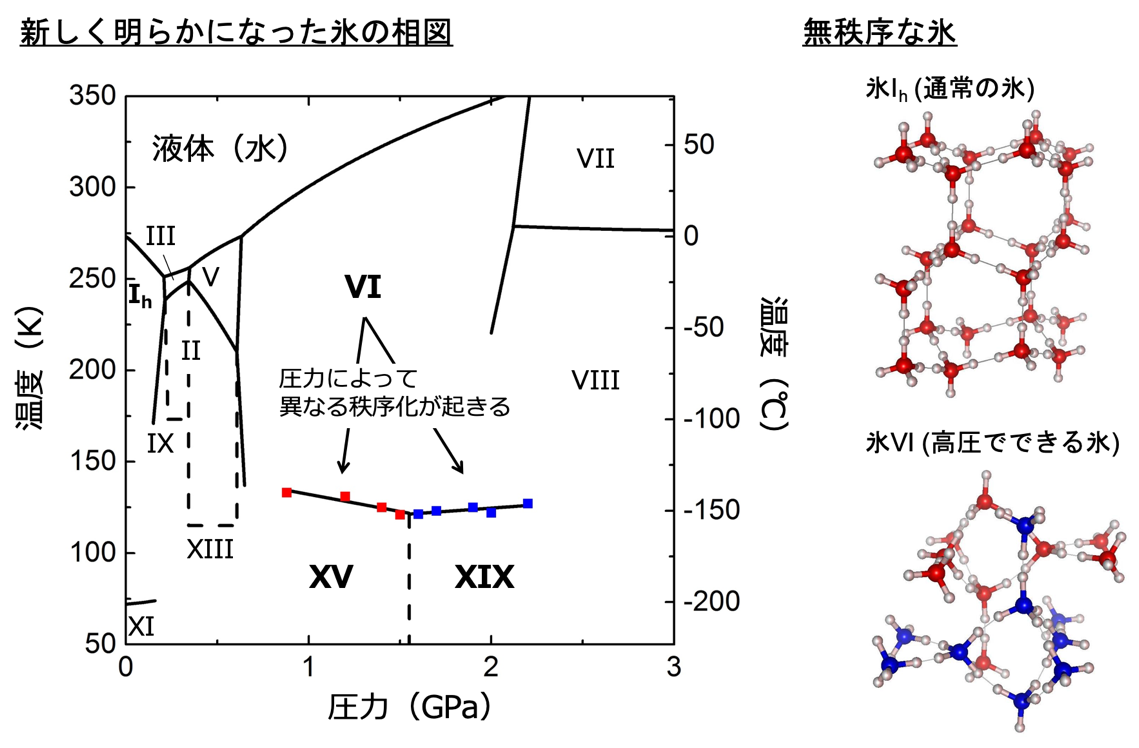
Figure 1: Temperature-pressure phase diagram of ice newly revealed in this study and crystal structures of ice Ih ( normal ice) and ice VI with disordered orientation of water molecules. Red and blue dots in the phase diagram are experimental points for dielectric measurements. Dotted lines indicate experimentally unconfirmed phase boundaries. In the crystal structure of ice Ih, red circles represent oxygen atoms and white circles represent hydrogen atoms. In the crystal structure of ice VI, oxygen atoms are represented by red and blue, and water molecules grouped by each color form independent networks.
In ice VI, the oxygen atoms are neatly arranged in a periodic sequence, but the hydrogen atoms are arranged discretely, forming hydrogen bonds with two of the four neighboring water molecules. This disjointed hydrogen configuration (orientation of water molecules) is called the disordered phase. By lowering the temperature, the entire water molecules reorient in a specific direction to each other, resulting in a phase transition (ordering) to ice XIX. Examples of this new structure formed by the ordering of water molecule orientations are common in other polymorphs of ice as well. For example, ice Ih is a disordered phase, but when it is cooled to about -200°C, it becomes ice XI. Until now, it has been thought that there is only one way of ordering for each disordered phase. However, ice XIX is the second ordered phase of ice VI (the first ordered phase was ice XV (Note 5) ), showing for the first time that there are multiple ways of ordering ice. These two ordered phases have different stable regions depending on the pressure, and the ice XIX found in this study was found to form at higher pressure than ice XV. Furthermore, the crystal structure of ice XIX shows that the two ordered phases have different spatial inversion symmetries (Note 6 ), and differences in electrical and optical properties may also be discovered in future studies. Theoretical calculations have also indicated the existence of multiple ordered phases in other polymorphs, and it is expected that this discovery will lead to the discovery of further diversity in ice structure and physical properties.
This research result has been selected as an Editor's highlight inNature Communications.
Publication details
Research Background
Ice is probably the most familiar crystal to us humans. A crystal is a solid body in which atoms are periodically arranged in a regular pattern. There must be countless possible ways to arrange them, but have you ever wondered why one of them is realized? In many materials, only a very few crystalline structures are realized because one particular arrangement of atoms or molecules is energetically superior to the others. In the case of ice, on the other hand, there are many energetically close arrangements of water molecules. Therefore, many polymorphs with different crystal structures appear depending on temperature and pressure. Furthermore, in ice, there are also degrees of freedom in the direction of charge bias (dipole moment) of the water molecules arranged on the crystal lattice points, and different polymorphs can emerge depending on the ordering of the orientation of the water molecules. Since there are countless possible ways of this ordering, it is not difficult to imagine that there are many molecular arrangements that are energetically close to each other. However, the ice polymorphs discovered so far have only one ordered phase for one disordered phase, and the question of whether ice can have multiple ordered phases has been a major issue. Against this background, it was pointed out in 2018 that there may be another ordered phase in the ice high-pressure phase, ice VI (disordered phase), besides the known ordered phase, ice XV. However, there was no direct experimental evidence for the existence of this second ordered phase, and researchers were divided over its existence.
Research Details
To observe the phase transition to the ordered phase of ice VI and to show that this ordered phase is different from the previously reported phase of ice XV, neutron diffraction experiments that can provide detailed information on the hydrogen configuration must be performed under the special conditions of low temperature and high pressure (Note 2). The main reason why the second ordered phase of ice VI has not been directly observed until now is that it is technically difficult to perform neutron diffraction experiments under these low temperature and high pressure conditions. On the other hand, the movement of water molecules in ice and their ordering can also be observed as a change in dielectric constant (Figure 2 (Note 1 )). Although dielectric measurements do not reveal the crystal structure, the detection of ordering is more sensitive than neutron diffraction measurements. Therefore, we first performed a comprehensive temperature-pressure phase study of ice VI ordering by dielectric measurements. In this study, we also introduced a newly improved high-pressure cell for dielectric permittivity measurements (Figure 2).
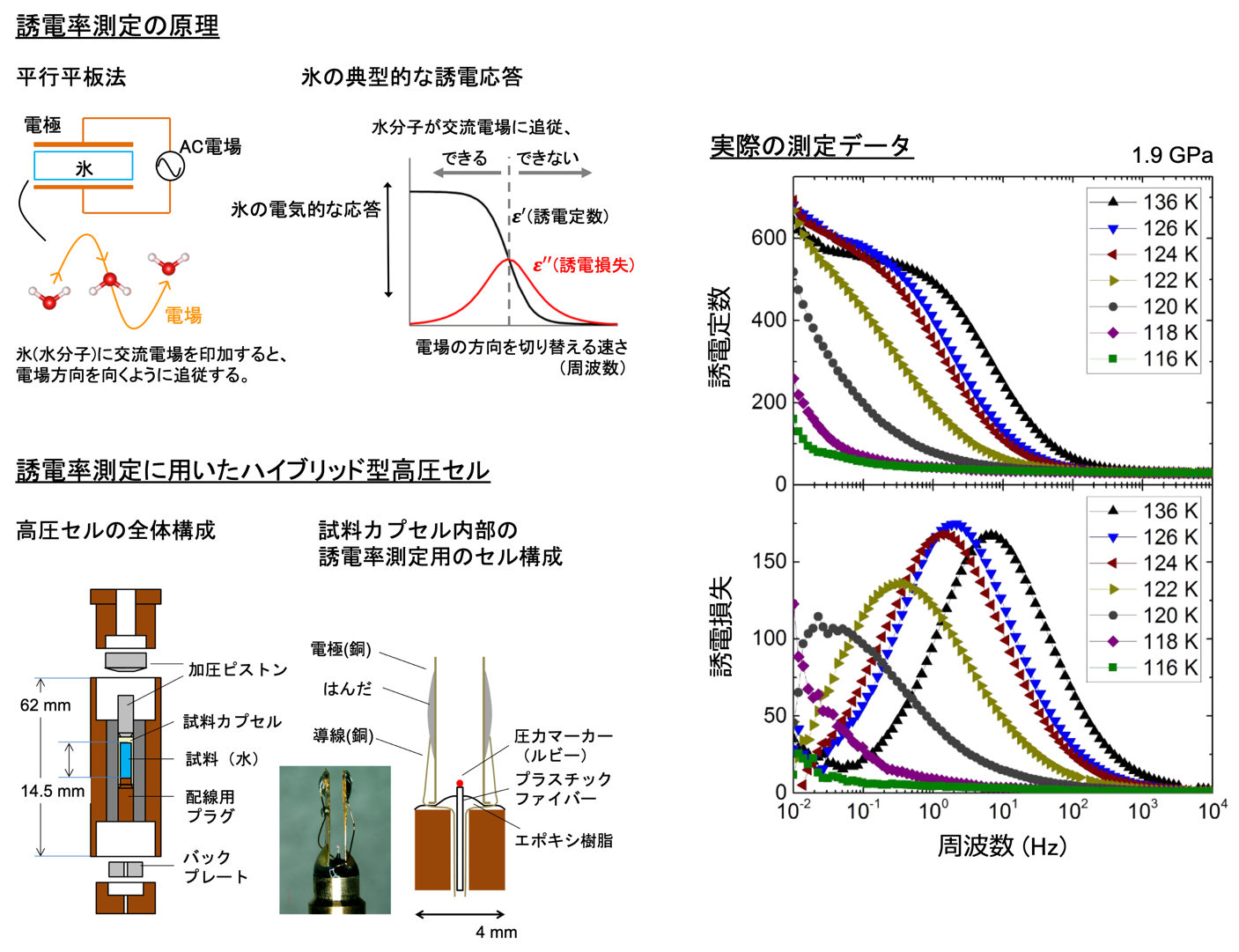
Figure 2: Illustration of the principle of dielectric constant measurement, examples of actual measurement data, and the structure of the newly developed hybrid-type high-pressure cell for dielectric constant measurement. The measured data shows that the dielectric response dramatically decreases near 124 K, indicating that ice ordering has occurred (dielectric constants and losses are shown divided by the dielectric constant of vacuum).
The advantage of the improved cell is that the pressure of the sample can be measured simultaneously along with the dielectric constant. Plotting the ice VI ordering temperature on the phase diagram reveals that the gradient of the phase boundary turns from negative to positive on the low and high pressure sides after around 1.6 GPa (Figure 1). This indicates the emergence of different ordered phases in these two regions. By comparing these results with previous results, it was found that the change in dielectric constant on the low-pressure side suggests a phase transition from ice VI to ice XV, and the change on the high-pressure side suggests a phase transition from ice VI to a new ordered phase, respectively. Therefore, the research group decided to conduct powder neutron diffraction experiments in the pressure region above 1.6 GPa by using a temperature and pressure tunable apparatus for neutron diffraction (commonly known as the Mito system (Note 2) ), which was originally developed by the group. The experiment was conducted at the high-pressure beamline PLANET (Note 7) in the Materials and Life Science Experimental Facility at J-PARC. As a result, we found that there are several peaks in the diffraction pattern obtained from the ice XIX crystal structure that cannot be explained by ice XV (Figure 3).
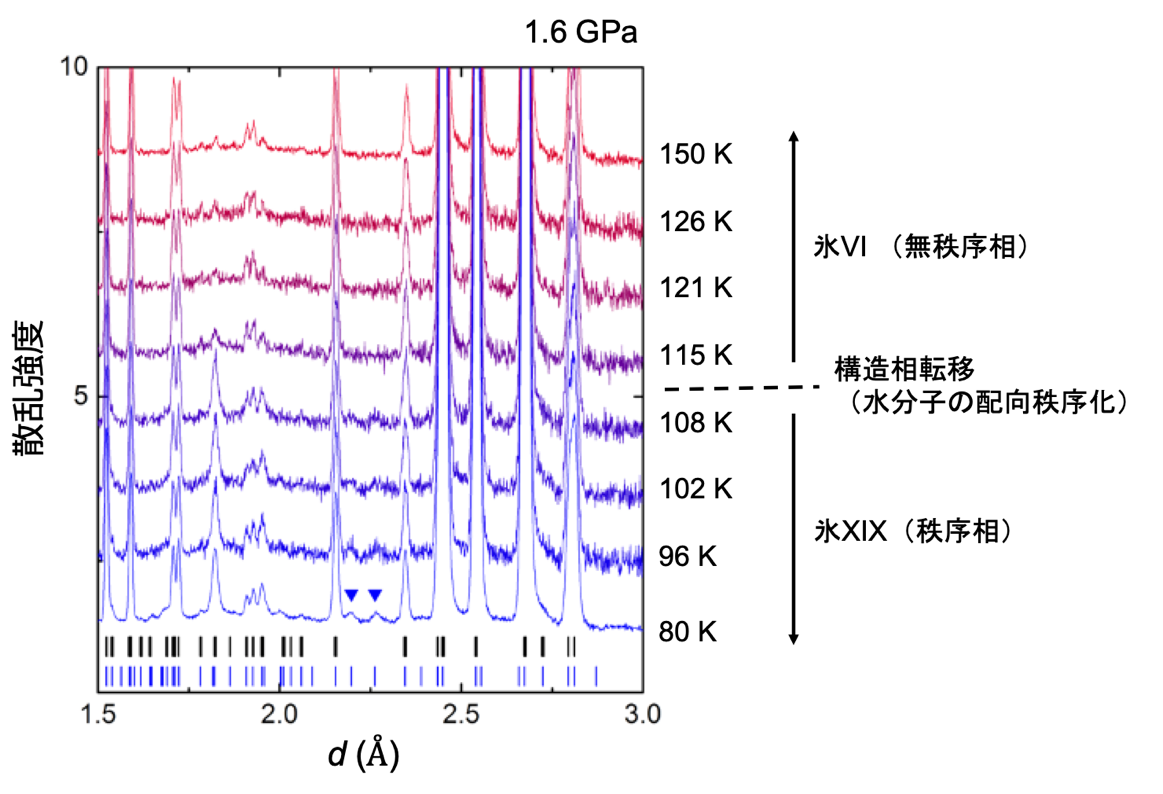
Figure 3: Diffraction patterns of ice VI and XIX measured by powder neutron diffraction experiments under high pressure; the diffraction pattern changes between 115 K and 108 K due to the phase transition. In particular, the two small peaks represented by blue inverted triangles are diffraction peaks that cannot appear in ice VI or in the known ordered phase XV (the black and blue lines at the bottom of the pattern indicate the positions of diffraction peaks that can appear in the crystal structures of ice VI, XV and ice XIX, respectively).
These peaks can be explained by considering that the unit lattice of ice XIX is larger than that of ice XV (√2 × √2 × 1x). Thus, this is the first experimental confirmation that a second hydrogen-ordered phase does indeed exist in ice VI. Since a new Roman numeral is assigned to each new ice polymorph, this second ordered phase becomes ice XIX. Detailed structural analysis has led to the proposal of two structures for the crystal structure of ice XIX that do not have space-reversal symmetry. In one of these structures, the charge bias of the water molecules is not canceled throughout the crystal structure (Fig. 4). This macroscopic charge bias may provide ice with functionalities such as ferroelectricity (Note 6).
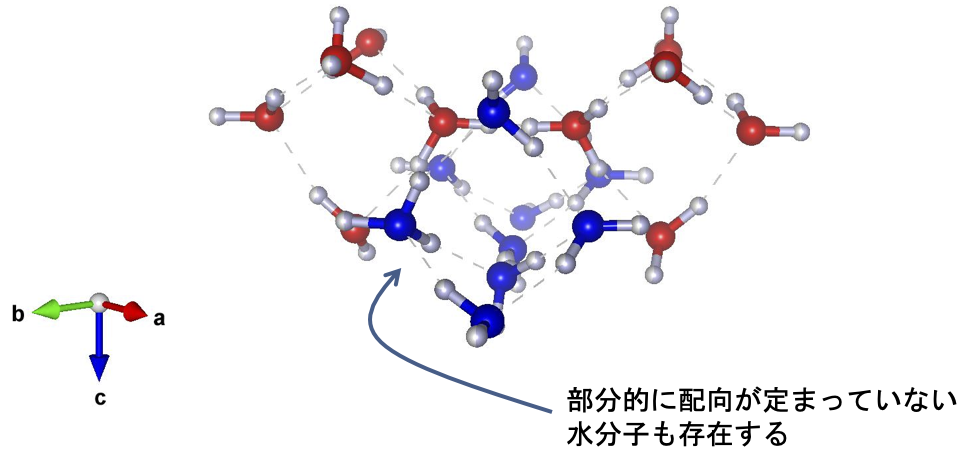
Figure 4: One of the crystal structures of the proposed ice XIX. The two water molecule networks (red and blue), which are characteristic of the crystal structure of ice VI, are retained, while the orientation of the water molecules is ordered. However, some water molecules remain partially disordered, and this is not a completely ordered phase of ice. The vertical direction ( c-axis ) in the paper is the polarization axis.
Significance of the Study
This research has revealed for the first time that the disordered phase, ice VI, has multiple ordered phases. This discovery is expected to lead to further research to search for second and third ordered phases in other disordered phases, and the number of polymorphs in ice is expected to increase further in the next few years. In such studies, it is a very interesting topic how conditions can be changed to induce new ordered states. Pressure is a powerful parameter that controls molecular orientation, as in this case, but new ordered states may be found in the future by controlling temperature, the electric dipole moment of water molecules, and the electric field that couples electrostatically with the dipole moment. This raises the prospect of a new development: the deliberate control of the ordering mode of ice. The rich electrical properties produced by water molecules are expected to lead to ideas for ultimately clean ice-based devices.
Finally, an important aspect of ice research is the mode of existence of ice in nature. In fact, as with many other polymorphs of ice, it is unlikely that ice XIX will actually be observed in nature, since a pressure of 1 GPa is equivalent to the pressure at a depth of about 30 km below the surface of the Earth, and such a deep temperature is too high for ice XIX to be stable at temperatures below -150 °C. However, the temperature of such a deep part of the earth is too high. However, just recently, ice VII, which was previously thought to not exist in nature, was found in a natural diamond inclusion. This fact tells us that we cannot assert that ice XIX does not exist anywhere in nature based on current knowledge alone. The temperature and pressure range in which ice XIX can exist stably was determined from dielectric measurements, and if ice XIX is ever found in nature in the future, the results obtained in this study will be the key to understanding its formation process. Even if ice XIX does not exist in nature, the stability region of each ice polymorph is important from the viewpoint of understanding ice itself.
Ice is one of the most studied materials because it is the most familiar crystal, yet new facts are still being discovered, such as the discovery of ice XIX. This is a good example of how what we know about matter is just the tip of the iceberg.
Journal
-
Journal name Nature Communications Title of paper Experimental evidence for the existence of a second partially-ordered phase of ice VI Author(s) Ryo Yamane, Kazuki Komatsu, Jun Gouchi, Yoshiya Uwatoko, Shinichi Machida, Takanori Hattori, Hayate Ito, Hiroyuki Kagi DOI Number 10.1038/s41467-021-21351-9 Abstract URL https://www.nature.com/articles/s41467-021-21351-9
Terminological Explanation
Note 1: Dielectric constant measurement device and change in dielectric constant due to ice ordering.
Dielectric constant is a physical quantity that expresses the ease with which electric charges accumulate when an electric field is applied to a material. In this study, as shown in Figure 2, a sample is placed between two electrodes, which are enclosed in a pressure cell. An alternating electric field of various frequencies was applied between these electrodes to measure the dielectric constant of ice under high pressure. Water has a relatively high dielectric constant, but when an electric field is applied to ice, the electric dipole moment of the water molecules responds in the direction of the electric field, which causes an accumulation of electric charge. When ordering occurs, the dielectric response is dramatically reduced because the water molecules are oriented in a fixed direction and cannot easily move from that direction. ↑up
Note 2: "Mito system," a variable temperature and pressure apparatus (high-pressure cell) for neutron diffraction and neutron diffraction experiments
Neutron diffraction is a structural analysis method that uses the diffraction phenomenon caused by neutron atoms. Neutrons interact with nuclei in atoms, and thus provide different information than X-rays, which interact with electrons. For example, in the case of X-ray diffraction, the scattering intensity is stronger for elements with large atomic numbers, i.e., those with a large number of electrons, while the scattering intensity is weaker for light elements with a small number of electrons, making them less visible. In particular, the scattering from hydrogen (protons), which have lost electrons due to the formation of covalent bonds, is extremely weak, making it difficult to accurately determine the position of protons by X-ray diffraction. On the other hand, neutron diffraction is often used to determine the structure of materials containing hydrogen because the scattering intensity is almost the same for light to heavy elements.
In this study, we used a variable temperature and pressure apparatus called the "Mito system" to perform neutron diffraction experiments at low temperatures and high pressures. This system has been developed by our research group since around 2009, and its main feature is that the temperature of the sample can be changed efficiently by insulating the area near the sample from the press itself (for details, see https://www.s.u-tokyo.ac.jp/ja/press/2020/6686/ for details). ↑upNote 3: Counting Ice Polymorphs and Ice Ih
Normal ice has a hexagonal (hexagonal) symmetry and is called ice Ih. A similar structure, but with cubic symmetry (ice Ic ), is formed when the layers of water molecules are stacked in a different way.
(see https://www.s.u-tokyo.ac.jp/ja/press/2020/6686/参照 for more information). Since these two can be regarded as different polymorphs, ice XIX is the 20th ice polymorph. ↑upNote 4 GPa order
A pascal (Pa) is a unit of pressure. For example, atmospheric pressure is about 100 kPa, which corresponds to a weight of 10 g per mm2, and 1 GPa is 10,000 times that, which corresponds to a weight of 100 kg per mm2. *Corrected on April 18,2024 ↑up
Note 5 Ice XV
This is one of the ordered phases caused by the ordering of water molecules in the high-pressure phase of ice, ice VI, which was first reported in 2009. In ice XV, not all water molecules are oriented in the same way, and some water molecules remain randomly oriented (Figure 4), so strictly speaking, ice XV should be called a "partially ordered phase" (for details, see https://www.s.u-tokyo.ac.jp/ja/press/2016/4927/ for details). Ice XIX discovered in this study is also a partially ordered phase, but in this press release, we simply refer to it as the ordered phase for the sake of clarity. ↑up
Note 6 Spatial inversion symmetry
When the position of any atom ( x, y, z ) in a crystal is manipulated to be ( -x,-y, -z ), the crystal structure is said to have spatial inversion symmetry if it is unchanged before and after the manipulation. In a material lacking this symmetry, for example, positive and negative charge bias may spontaneously occur on the front and back sides of the crystal, and this bias may be controlled by an electric field or stress (a material in which positive and negative charges can be switched by an electric field is called ferroelectric material, and a material in which the size of charge bias can be changed by stress is called piezoelectric material). Therefore, one important guideline for exploring the functionality of materials is the presence or absence of spatial inversion symmetry. ↑up
Note 7: High-pressure beamline PLANET at the Materials and Life Science Experimental Facility in J-PARC
J-PARC is an abbreviation for Japan Proton Accelerator Research Complex, which is jointly operated by the Japan Atomic Energy Agency and the High Energy Accelerator Research Organization. It consists of a proton accelerator group and a group of experimental facilities: the Materials and Life Science Experimental Facility, the Neutrino Experimental Facility, and the Hadron Experimental Facility, where research in a wide range of fields including materials science, life science, particle physics, and nuclear physics is conducted. In this study, experiments were conducted using the ultrahigh-pressure neutron diffractometer PLANET at the Materials and Life Science Laboratory; PLANET is a neutron diffractometer specialized for high-pressure, and is indispensable for this study because it can obtain sufficiently accurate data under low temperature and high pressure. ↑up


