DATE2024.09.26 #Press Releases
Scientists discover a single-electron bond in a carbon-based compound
—The discovery of a stable single-electron covalent bond between two carbon atoms validates a century-old theory—
Summary
Researchers led by Takuya Shimajiri (at the time of research: Hokkaido University, currently: project assistant professor at the University of Tokyo) and Associate Professor Yusuke Ishigaki of Hokkaido University have made the first-ever observation of a stable, single-electron carbon-carbon covalent bond. This type of single-electron sigma bond was hypothesized in the early 1930s by none other than the Nobel laureate Linus Pauling. A few observations have been made since then, but never in carbon or hydrogen. The team of researchers achieved this breakthrough by oxidating a derivative of hexaphenylethane in the presence of iodine. They studied the resulting crystals using X-ray diffraction analysis and confirmed the presence of the covalent bond by Raman spectroscopy, a form of chemical analysis. The discovery deepens our understanding of chemical bonding theories and provides further insights into chemical reactions. The findings were published in the journal Nature.
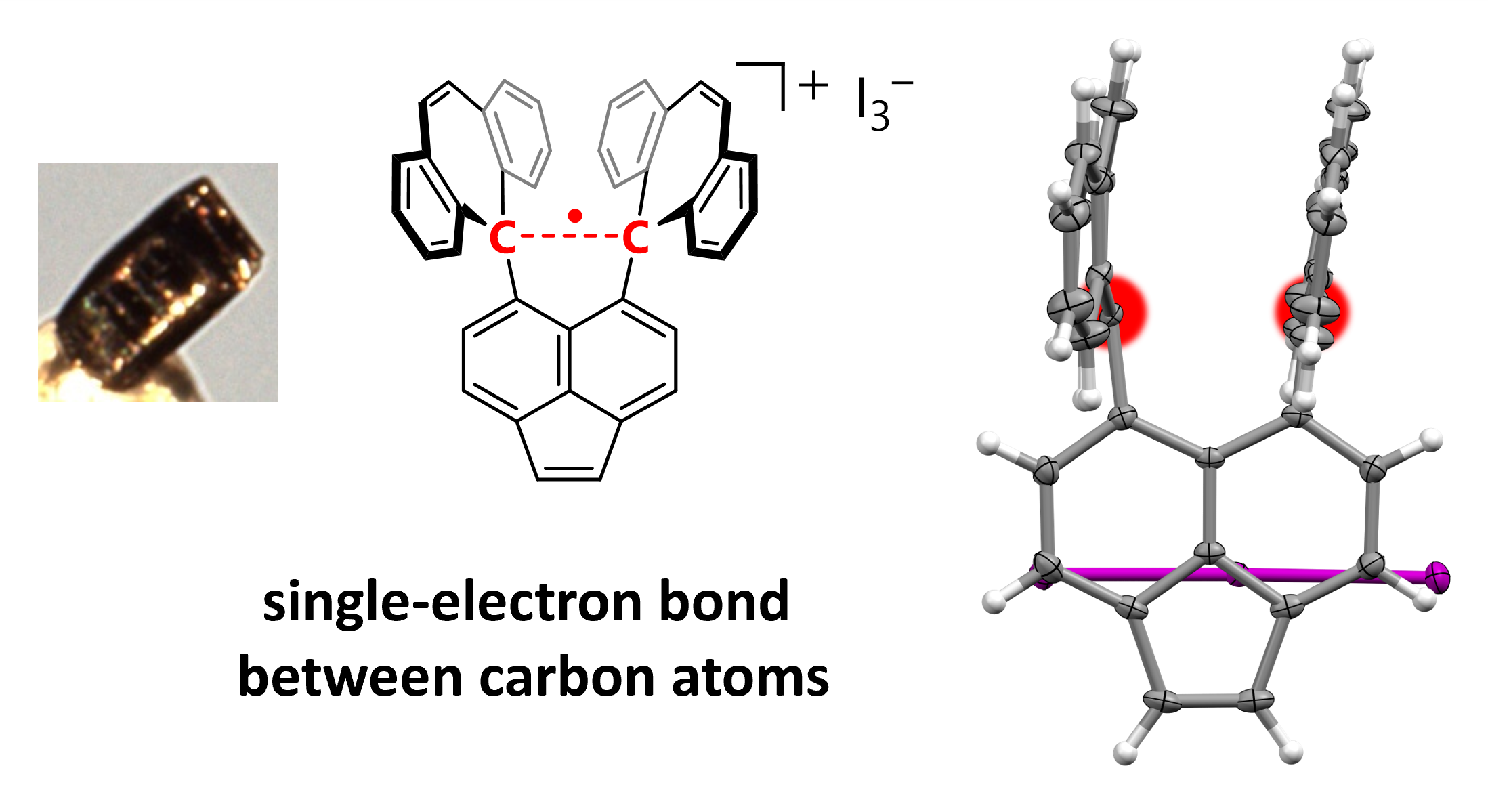
Figure:Structure of the compound highlighting the C–C one-electron sigma bond (red). (Takuya Shimajiri, et al. Nature. September 25, 2024)
Please visit Hokkaido University’s website for the full press release.
Journal
-
Journal name NatureTitle of paper


