DATE2024.08.03 #Press Releases
Development of a self-repairing catalyst
Innovative technology to improve catalyst lifetime
Summary
A research group led by Assistant Professor Taku Kitanosono and Professor Shu Kobayashiof the Graduate School of Science at the University of Tokyo has successfully developed a catalyst that can spontaneously repair itself without the addition of external energy during the reaction. This catalyst is deemed to be a “self-repairing catalyst”. Chiral Lewis acid catalysts have played an important role in organic synthesis due to their high reactivity and ability to control selectivity, but catalytic degradation due to hydrolysis of metal ions has been an issue. In particular, catalysts with high Lewis acidity are at high risk of hydrolysis due to their strong interaction with water molecules, and therefore, improved stability has been sought.
In this study, these issues were overcome by molecular design based on the zwitterionic structure. The designed catalyst exhibited high stability both theoretically and experimentally, and hydrolysis was suppressed even under basic conditions due to its buffering effect.
This self-repairing, highly active, and highly selective catalyst is expected to contribute to the reduction of environmental impact and the development of highly efficient synthetic processes. Furthermore, this small molecule-based strategy has the potential to be applied to other metal-catalyzed reactions, and the results are noteworthy as they open a new door to catalytic science.
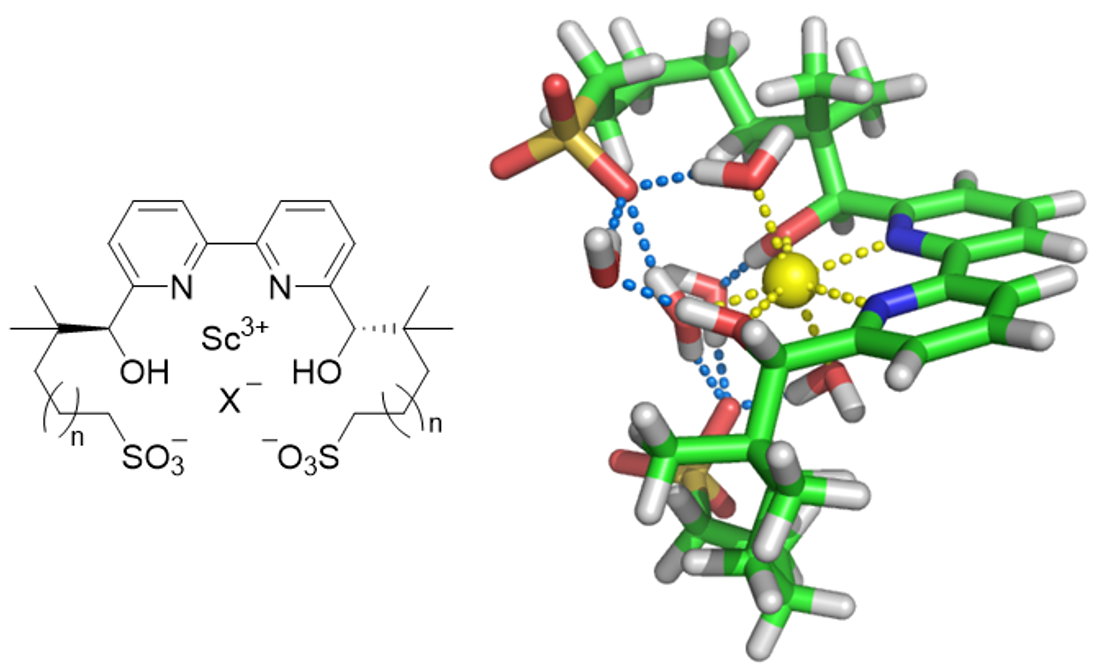
Figure : Basic framework of the designed scandium complex (left figure) and its structural model in water (right figure; for n = 3)
Journals
-
Journal name Journal of the American Chemical Society Title of paper


