DATE2024.06.19 #Press Releases
Elucidating the structure of the QRFP receptor, which regulates metabolism and appetite
Summary
A group led by Professor Osamu Nureki at the University of Tokyo in collaboration with Professor Asuka Inoue at Tohoku University has determined the steric structure of the signaling complex between the QRFP receptor (GPR103) activated by the endogenous peptide QRFP and trimeric G protein Gq by cryo-EM single particle analysis. QRFP is a peptide hormone that plays an important role in the regulation of energy metabolism and appetite by activating its receptor, GPR103. However, the detailed mechanism of how QRFP activates GPR103 was unknown. In this study, based on the determined structure, we clarified the binding mode of QRFP and the activation mechanism of the receptor, and found that QRFP has a unique binding mode and dynamics spanning the transmembrane to extracellular regions of GPR103 and can bind to the receptor with high affinity. The results of this study will enable the design of more effective and safer therapeutics targeting the QRFP-GPR103 system and will contribute to the development of treatments for metabolic and appetite disorders.
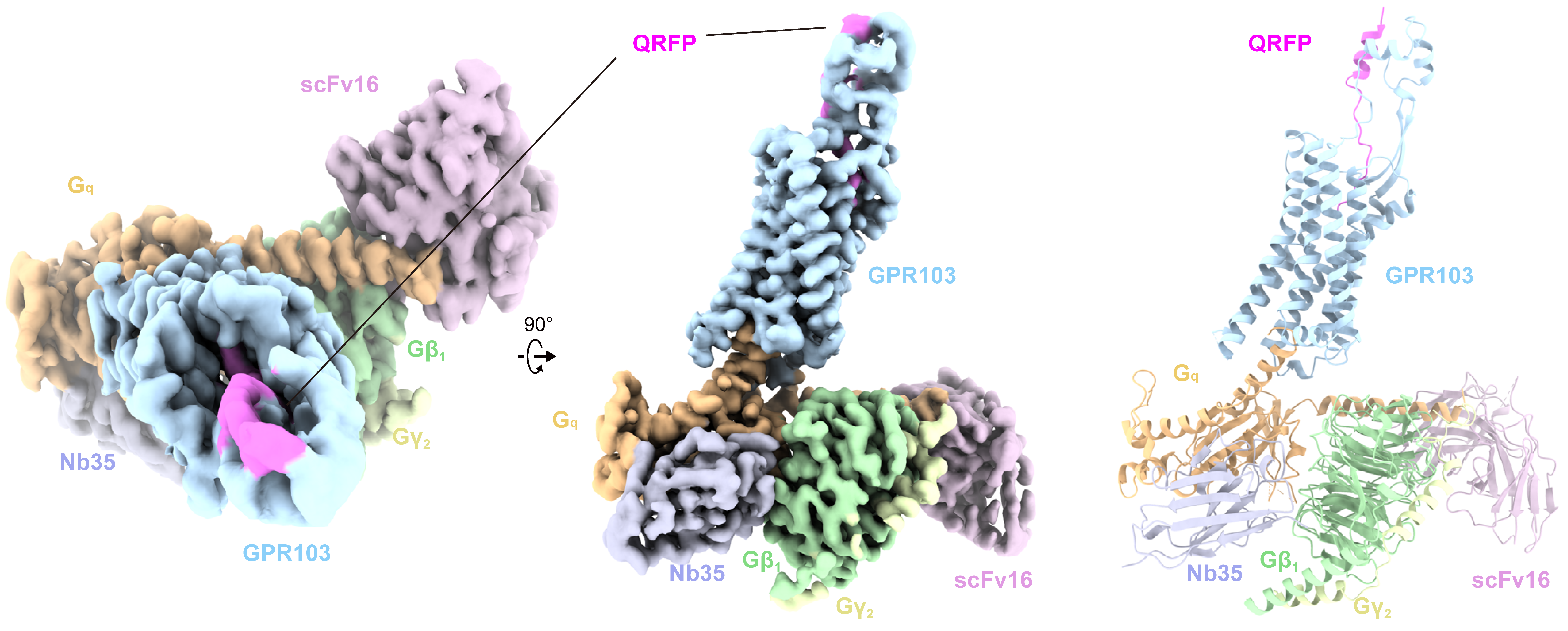
Figure : Overall structure of GPR103-Gq complex
Links:
Tohoku University
Journals
-
Journal name Nature Communications Title of paper


