DATE2024.05.21 #Press Releases
A new platform for highly selective reactions in vivo
--Chiral Lewis acid catalysts based on nanoscale metal-organic frameworks (MOFs)--
Summary of Presentations
A research group led by Professor Shū Kobayashi and Assistant Professor Taku Kitanosono at the Graduate School of Science at the University of Tokyo has successfully explored new possibilities for Lewis acid catalysis, representing an important step toward the in vivo application of organic synthesis techniques that have been practiced in flasks.
In modern organic chemistry, Lewis acid catalysts play a crucial role due to their unique reactivity and ability to control selectivity. However, classical Lewis acids are sensitive to moisture and decompose in the presence of water. Furthermore, Lewis acids are easily poisoned in vivo by Lewis base functional groups such as amines and thiols in proteins and small biological molecules. These limitations have constrained the potential applicability of Lewis acid catalysts.
In this study, we aimed to overcome these issues by designing chiral Lewis acid linkers and metal-organic framework (MOF) technology. Catalyst development in vivo has been mainly based on precious metal catalysts, which are relatively resistant to poisoning, but in this study, Lewis acid catalysts were successfully made to function in the presence of proteins.
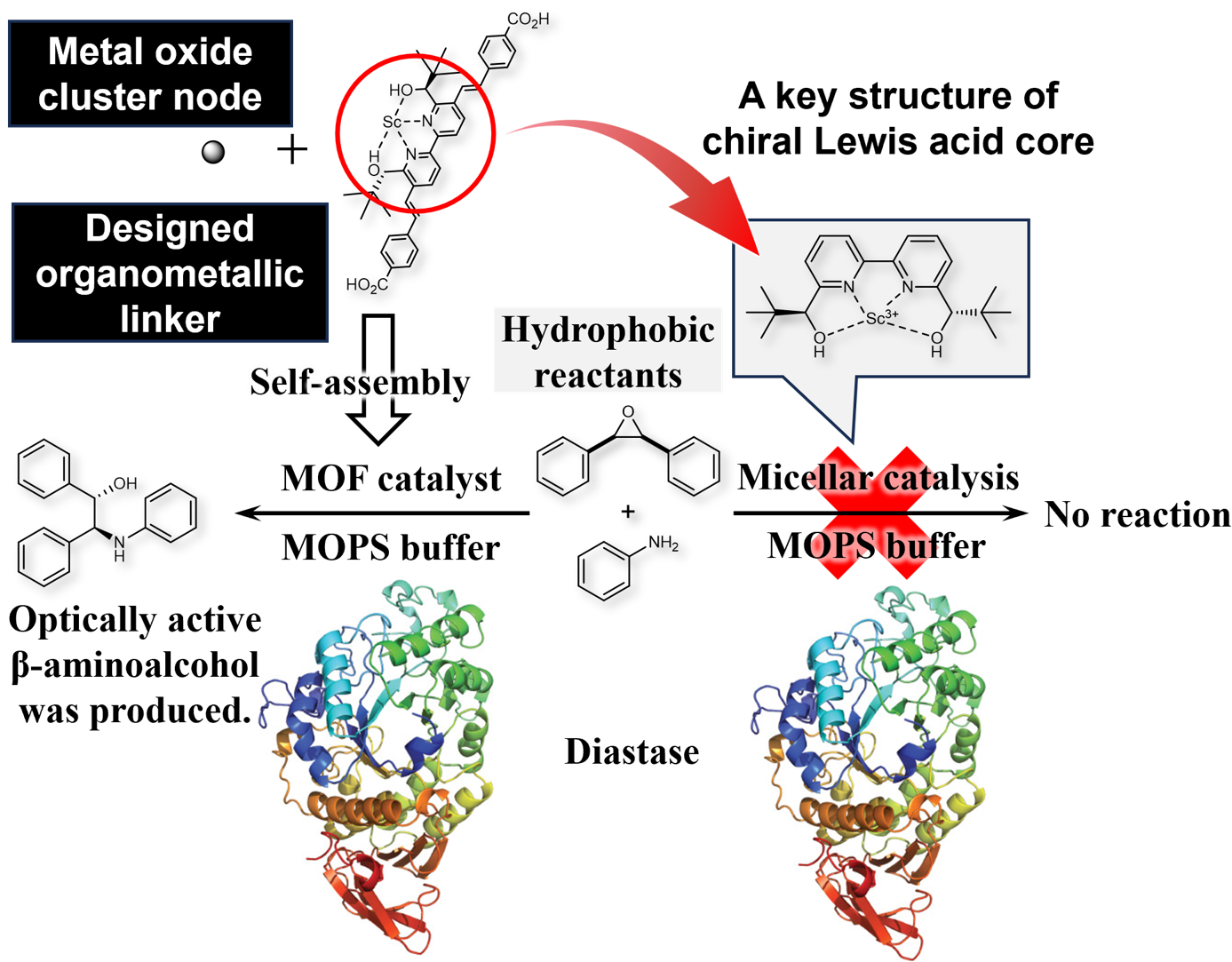
Figure : Catalyst poisoning experiments with proteins
Journals
-
Journal name Chemical Science Title of paper


