DATE2023.04.05 #Press Releases
Elucidation of the neural mechanisms underlying the 24-hour cycle of odor sensitivity
Disclaimer: machine translated by DeepL which may contain errors.
Shunsuke Takeuchi (Doctoral student at the time of research)
Kimiko Shimizu (Assistant Professor at the time of research)
Yoshitaka Fukada, Emeritus Professor
Kazuo Enomoto, Professor, Deputy Director and Senior Staff, International Research Center for Neurointelligence
Key points of the presentation
- We found that olfactory sensitivity to olfactory substances fluctuates within a 24-hour cycle, with higher sensitivity during the night, when mice are active, and lower sensitivity during the day, when mice are at rest.
- This diurnal variation in olfactory sensitivity is generated by oscillations in the expression of the biological clock, which is responsible for the regulation of the expression of several genes involved in neural activity and neurotransmitter release.
- The results of this study are expected to lead to the elucidation of the mechanism by which various brain functions in animals are variably regulated in an approximately 24-hour cycle.
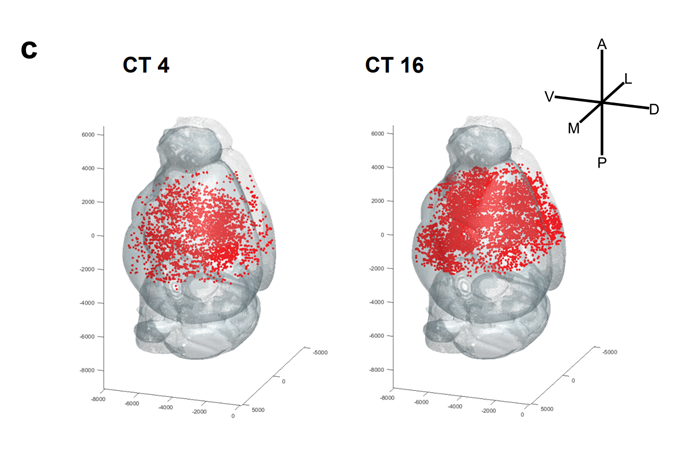
Distribution of odor-responsive neurons during the day (CT4) and at night (CT16)
Summary of presentation
Shunsuke Takeuchi, Graduate Student, Assistant Professor, Kimiko Shimizu, Professor, Yoshitaka Fukada, and Kazuo Enomoto, Graduate School of Science, The University of Tokyo, have focused on the neural activity of the olfactory circuit of mice, and by manipulating clock genes specifically for each neuron in the olfactory circuit, they have shown that (1) the diurnal variation in neural activity in the olfactory circuit is mainly (2) The biological clock of the olfactory circuit transcriptionally regulates the expression level of genes involved in neuronal activity in a 24-hour cycle.
The biological clock (Note 1) that controls the life rhythms of organisms exists in all cells that make up the body, but in many organisms, it is thought that some neurons in the brain sense and transmit information from the outside world, thereby synchronously driving the biological clock in the body. On the other hand, recent studies have reported that the biological clock in the body continues to oscillate at a constant rhythm even when the central clock in the brain does not work, and the role and importance of the biological clock in individual cells is attracting attention. However, because it has been technically difficult to manipulate a group of genes called clock genes, which are components of biological clocks, in a specific group of cells, there are still many aspects of cell-specific biological clock functions that remain unclarified.
It is hoped that this research will lead to the elucidation of the mechanism by which the biological clock in neurons generates diurnal variation in brain function through transcriptional regulation of genes.
Contents of Presentation
On our planet Earth, the external environment surrounding living organisms, such as temperature and light, changes on a 24-hour cycle. In order to adapt to such external environment, many organisms have biological clocks that can control their internal activities on a 24-hour cycle, and can vary their biological functions, such as activity patterns and responsiveness to the external environment, within a 24-hour cycle. In mammals such as humans and mice, the brain region called the suprachiasmatic nucleus (Note 2) is thought to be the center of biological clocks and is thought to integrate and control biological clocks in other brain regions and peripheral tissues. On the other hand, recent studies have reported cases in which biological clocks in the body continue to oscillate in a coordinated manner even in animals in which the suprachiasmatic nucleus has been surgically removed, and the role and importance of biological clocks unique to each tissue and cell is attracting attention. However, since it has been technically difficult to separate the central clock in the suprachiasmatic nucleus from the clocks in other cells and tissues and manipulate them experimentally, the biological significance and control mechanisms of tissue- and cell-specific biological clocks remain largely unresolved.
Focusing on the olfactory response and neural activity of the olfactory circuit in mice, this research group has been studying the biological significance and regulatory mechanisms of the biological clock specific to the olfactory circuit. First, they conducted experiments in which odorants were presented to mice at various times of the day, and found that the neural activity of the olfactory circuit, like the olfactory response, shows diurnal variation, being higher during the night than during the day. Furthermore, when the suprachiasmatic nucleus, the center of the biological clock, was inactivated, the neural activity of the olfactory circuit fluctuated during the day as in normal individuals, suggesting that the diurnal variation in the olfactory response may be controlled by mechanisms other than the central clock in the suprachiasmatic nucleus. By using several genetically modified mice and the adeno-associated virus vector (*3), the research team succeeded in deleting the clock genes specifically in each neuron in the olfactory circuit without affecting the central clock in the suprachiasmatic nucleus.
The mouse olfactory circuit is composed of five types of neurons in three main regions (Fig. 1). The results showed that when the clock gene was specifically deleted in the pyramidal neurons of the pisiform cortex (Note 4), which is the region of the brain responsible for olfactory information processing, the diurnal variation of neural activity was not observed. (Note 4), a region in the brain that is responsible for olfactory information processing. This indicates that the diurnal variation in neural activity in the olfactory circuit is generated by the biological clock activity of the neurons that make up the olfactory circuit. Furthermore, we analyzed the function of clock genes in pyramidal cells of the pisiform cortex and found that they transcriptionally regulate the expression of multiple genes involved in neuronal activity and neurotransmitter release. These results indicate that the neuronal activity of the mouse olfactory circuit is regulated by the clock genes in the piriform cortex and that the clock genes in the piriform cortex may regulate the activity of the olfactory circuit on a circadian basis through the regulation of expression of multiple genes involved in neuronal activity (Figure 2).
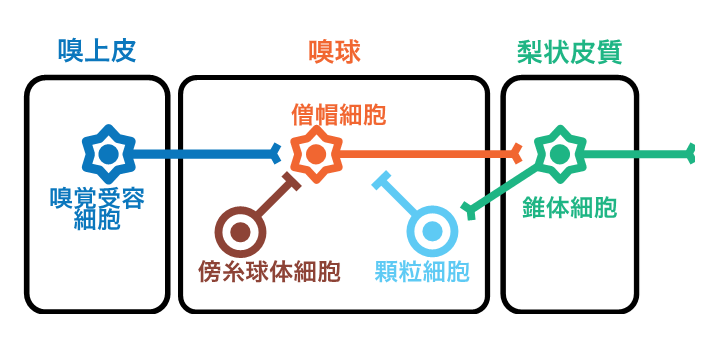
Figure 1: Mouse olfactory circuit
The mouse olfactory circuit is composed of the olfactory epithelium, which contains odorant receptor cells, the olfactory bulb, which contains mitral cells and two types of inhibitory neurons (paraglomerular cells and granule cells), and the pisiform cortex, which is a cortical region and contains pyramidal cells.
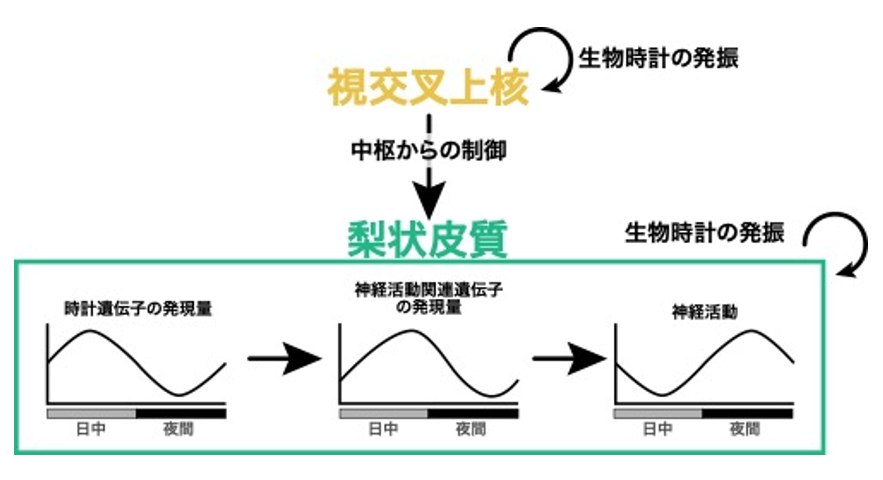
Figure 2: Working hypothesis of biological clock control mechanisms in the pisiform cortex revealed in this study.
In the pisiform cortex, clock genes that constitute the biological clock oscillate in a central, suprachiasmatic nucleus-independent manner and are thought to generate diurnal variations in the expression levels of neural activity-related genes and neural activity. On the other hand, the suprachiasmatic nucleus is thought to facilitate biological clock entrainment by regulating the pisiform cortex.
Recent studies have reported that various brain functions, such as memory, are subject to fluctuating control in a 24-hour cycle, but the mechanisms and physiological significance of this phenomenon are unknown. It is hoped that the results and methods of this study will deepen our understanding of the mechanisms and physiological significance of autonomous daily cycle fluctuations of specific neurons and neural circuits.
Publication Information
-
Journal Title Communications Biology Title of paper The circadian clock in the mouse piriform cortex plays an intrinsic role in daily changes in odor-evoked neural activity Author(s): Shunsuke Takeuchi, Kimiko Shimizu, Yoshitaka Yoshitaka Shunsuke Takeuchi, Kimiko Shimizu, Yoshitaka Fukada, and Kazuo Emoto* (author) DOI Number
Research Grant
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan, Grant-in-Aid for Scientific Research on Innovative Areas "Dynamic Control of Brain Functions by Scrap and Build" (PI: Kazuo Enomoto), JSPS Basic Research (A) "Molecular Cellular Basis for Organization, Maintenance and Management of Neural Circuits" (PI: Kazuo Enomoto), Japan Medical Research and Development Organization The research was supported by AMED-CREST, "Elucidation of Life Phenomena in Early Life Stages for the Improvement of Health and Medical Care" (Principal Investigator: Kazuo Enomoto).
Terminology
1 Biological clock
A timekeeping mechanism in the body of living organisms that has a cycle of approximately 24 hours, independent of changes in the external environment. 2017 Nobel Laureates in Physiology or Medicine Jeffrey Hall, Michael Rosbash, and Michael Young, among others, have shown that biological clocks are mainly composed of multiple transcription factors The biological clock is composed mainly of multiple transcription factors. ↑
Note 2: Suprachiasmatic nucleus
The suprachiasmatic nucleus is a brain region named the suprachiasmatic nucleus because it is located directly above the optic chiasm, the point where the optic nerves cross in mammals. Surgical removal of the suprachiasmatic nucleus causes the loss of diurnal variation in activity patterns and disruption of the phase of the biological clock in the elimination, suggesting that it is a brain region that plays a central role in the biological clock in mammals. ↑up
Note 3 Adeno-associated virus vector
A single-stranded DNA virus of the parvoviridae family and non-pathogenic, the AAV vector derived from it is recognized as a highly safe genetic manipulation tool. Furthermore, since AAV vectors can infect both proliferating and non-proliferating cells, and can efficiently infect post-differentiated neurons to induce long-term gene expression, they have recently been used to label and manipulate brain neural circuits. ↑up
Note 4 Pearly cortex
The pisiform cortex is a brain region that constitutes the mammalian olfactory circuit and receives odor information following the olfactory epithelium and olfactory bulb, which receive odorant substances. It is thought that the neural activity patterns of pyramidal cells in the pisiform cortex encode odorant information. ↑


