DATE2023.02.17 #Press Releases
Elucidation of the function of translation factor eIF5A in atypical translation reactions
Disclaimer: machine translated by DeepL which may contain errors.
--The mechanism by which the peptide synthesis reaction is carried out without the need for mRNA--.
The University of Tokyo Institute of Medical Science
Graduate School of Science, The University of Tokyo
Graduate School of Frontier Sciences, The University of Tokyo
Summary of Presentations
A research group led by Professor Toshifumi Inada and Project Academic Specialist Shuhei Ebine at the Department of RNA Regulation, Institute of Medical Science, Graduate School of Science and Department of Computational Biology and Medical Sciences, Graduate School of Frontier Sciences, The University of Tokyo, and Professor Petr Tesina and Roland Beckmann at the Gene Center, University of Munich, has reported that the translation factor eIF5A The group led by Professor Petr Tesina and Professor Roland Beckmann of the Gene Center, University of Munich, has elucidated for the first time in the world that the translation factor eIF5A promotes the atypical translation CAT tailing reaction.
Ribosomes synthesize proteins by decoding the genetic code on mRNA and incorporating the corresponding tRNA. In normal protein synthesis reactions, the ribosome undergoes constant conformational changes, and the supply of an energy source called GTPase makes this reaction possible. In vivo, aberrant mRNAs, such as Nonstop mRNA, cause ribosome stagnation and collisions between ribosomes. However, the quality control mechanism quickly responds by dissociating the colliding ribosomes and inducing degradation of the peptide chain in the process of synthesis.
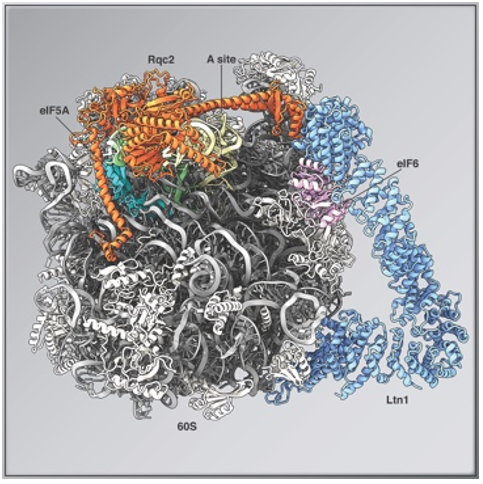
It is known that Rqc2, a quality control factor, binds to the large subunit of the dissociated ribosome and adds a special sequence called the CAT tail to the C-terminal side of the protein during synthesis, despite the absence of mRNA or GTPases. Although physiological functions of the CAT tail, such as promoting degradation of proteins in the process of synthesis and the CAT tail itself acting as a marker for degradation, have been analyzed, the molecular mechanism of the CAT tailing reaction has not been fully understood.
By structural analysis using cryo-EM and functional analysis using genetic methods, we found that eIF5A plays a role as a promoter of the CAT-tailing reaction, and that binding of eIF5A triggers a conformational change of the ribosome, which is essential for the CAT-tailing reaction. This achievement is expected to lead to the elucidation of the pathogenic mechanism and the establishment of Drug Discovery Initiative for trans-muscular diseases caused by abnormalities in the nervous system where translation is active, especially in neuromuscular muscles essential for muscle movement.
The research results were published in the U.S. scientific journal Molecular Cell on February 16.
For more information, please visit the website of the Institute of Medical Science, The University of Tokyo.


