DATE2022.04.15 #Press Releases
Super Catalyst for Nuclear Reduction Contributing to a Hydrogen Society
Disclaimer: machine translated by DeepL which may contain errors.
Osamu Kobayashi, Professor, Department of Chemistry
Hiroyuki Miyamura, Assistant Professor, Department of Chemistry
Key points of the presentation
- Hydrogenation of aromatic rings is an important reaction applicable to the storage and transport of hydrogen and the production of fine chemicals (pharmaceuticals, chemical products, agrochemicals, etc.). In this study, we found that a newly developed cooperative catalyst system for this reaction shows a reaction acceleration effect up to 30 times greater than that of the conventional method.
- This study discovered a significant increase in reaction rate and the development of new reactivity by using the cooperative catalytic system of heterogeneous metal nanoparticles and Lewis acid, leading to the development of new possibilities for cooperative catalysts using heterogeneous metal nanoparticles catalysts.
- This catalytic system is particularly active for aromatic compounds with sterically intricate multiple substituents and bulky, electron-rich aromatics, which have been particularly difficult to hydrogenate using conventional methods. It also showed high activity and selectivity for stereoselective hydrogenation of aromatic compounds with complex and bulky substituents, which require harsh conditions such as high temperature and high pressure in conventional methods, and synthesis of building blocks used as raw materials for bulk pharmaceuticals. This system is expected to provide a new method for highly efficient supply of useful substances.
Summary of Presentation
Hydrogenation reactions of aromatic rings such as benzene are applicable to hydrogen storage and transport, and are also important for the synthesis of functional molecules such as pharmaceuticals and bioactive substances. In this study, a heterogeneous rhodium-platinum bimetallic nanoparticle catalyst using an inexpensive organic-inorganic hybrid support and a catalyst system consisting of scandium triflate and ytterbium triflate, which are Lewis acids stable in water and alcohol solvents, were developed and applied to the hydrogenation of aromatic rings. hydrogenation of aromatic rings. This catalyst system works under mild conditions and can be used for aromatic compounds with multiple substituents and bulky, electron-rich aromatics, which are particularly difficult to hydrogenate using conventional methods. In the present study, we found that the catalyst system exhibited a reaction acceleration effect up to approximately 30 times greater than that of the conventional method. The catalyst system also shows high activity and selectivity for stereoselective hydrogenation of aromatic compounds with complex and bulky substituents, which requires harsh conditions such as high temperature and high pressure in the conventional method, and synthesis of building blocks for synthesis of bulk pharmaceuticals. These results can provide a new method for highly efficient supply of useful substances.
In this method, a chiral scandium complex is immobilized on a polystyrene backbone, and the product, catalyst, and water can be separated only by centrifugation operation. In the asymmetric ring-opening reaction of mesoepoxides, the catalyst and water could be reused 10 times, and the chiral 1,2-amino alcohols found in many pharmaceuticals were obtained efficiently. It was also applied to the asymmetric 1,4-addition reaction of thiols and the asymmetric Mukaiyama aldol reaction using formalin.
The research results were published online in the German chemistry journals "Angewandte Chemie" and "Angewandte Chemie Internationl Edition" at 0:00 a.m. Japan time (5:00 p.m. German time on April 14) on April 15, 2012. This research was conducted as part of a planned research project under the Grant-in-Aid for Scientific Research on Innovative Areas B (highly ordered catalytic chemistry realized by low-entropy reaction spaces) of the Grant-in-Aid for Scientific Research (MEXT) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Publication details
Background of Research
Hydrogen has been attracting attention in recent years as a clean energy source, and its effective utilization is expected in the field of synthetic organic chemistry, which supplies fine chemicals (fine chemicals) such as pharmaceuticals, chemical products, and agricultural chemicals. Among them, hydrogenation reactions of aromatic rings or aromatic rings containing heteroatoms are important for the transition to a hydrogen society, because they can be utilized not only in fine chemical synthesis but also in applications oriented toward hydrogen storage and transport using the organic hydride method (Note 1).
In complex compounds with various functions, such as pharmaceuticals, sterically intricate alicyclic moieties and heterocyclic moieties with various substituents often appear, and one of the most effective methods is to synthesize them by hydrogenation of the corresponding aromatic compounds. However, hydrogenation of aromatic compounds with multiple bulky substituents and bulky, electron-rich aromatic compounds is difficult and requires harsh reaction conditions such as high temperature and high pressure, and the development of an efficient synthetic method has been a challenge. Hydrogenation of aniline derivatives gives alicyclic compounds with nitrogen substituents, which are often found as synthetic intermediates of biologically active substances such as active pharmaceutical ingredients. However, hydrogenation of bulky aniline derivatives, such as N,N-dimethylaniline, requires harsh reaction conditions such as 20 atm hydrogen and 80 ºC or higher in the conventional method, and a more efficient catalytic system that can function under mild conditions is needed. In addition, the catalytic hydrogenation of ethylnicotinate, a nitrogen-containing aromatic compound, has been widely studied because its hydrogenated form provides an intermediate in the synthesis of bulk pharmaceuticals, but due to the stability of its partially hydrogenated form, high temperature and pressure conditions were required to obtain the saturated hydrogenated form.
On the other hand, a cooperative catalytic system in which multiple catalysts activate different substrates can synergistically reduce the activation energy of the transition state, making it possible to carry out highly difficult reactions under mild reaction conditions and with a small amount of catalyst. This is also attracting attention in achieving the SDGs, such as energy and material conservation. However, the development of such a cooperative catalyst system has been focused mainly on homogeneous catalysts, and the development of a cooperative catalyst system for heterogeneous catalysts, which can be recovered and reused, is still in its infancy, enabling further resource and material conservation.
Description of Research
In this study, we firstly developed a new cooperative catalyst system effective for hydrogenation of aromatic compounds with multiple bulky substituents, such as N,N-dimethylaniline and ortho-xylene. The Rh-Pt bimetallic nanoparticle catalysts on readily available organic-inorganic hybrid supports have shown very high catalytic activity in the hydrogenation of toluene under mild conditions such as ambient temperature and pressure, but the catalytic activity of the Rh-Pt bimetallic nanoparticles was not sufficient for the hydrogenation of aromatic compounds having more than one bulky substituent. However, the hydrogenation of aromatic compounds with multiple bulky substituents requires high temperature and pressure conditions. In this study, we found that the use of heterogeneous metal nanoparticles and Lewis acid as a catalyst system significantly increased the reaction rate of substrates that would be difficult to hydrogenate without Lewis acid, and in all cases, the hydrogenation of aromatic compounds proceeded in high yields under mild conditions of 40-50 °C reaction temperature and atmospheric hydrogen pressure. The hydrogenation reaction of aromatic compounds proceeded in high yields under mild conditions of 40-50 °C reaction temperature and atmospheric pressure hydrogen in all cases (Figure 1).
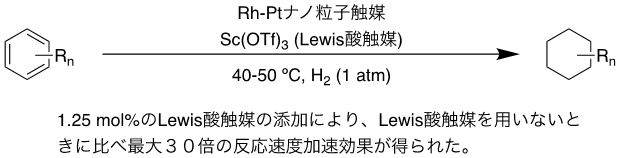
Figure 1: Hydrogenation of aromatic compounds using a catalytic system consisting of Rh-Pt nanoparticle catalysts and Lewis acid catalysts
The results of kinetic analysis and nuclear magnetic resonance (NMR) studies suggest that the Lewis acid interacts directly with the substrate in the liquid phase, and the reaction proceeds smoothly when the complex reacts with the hydrogen activated on the heterogeneous catalyst surface. . The kinetic analysis of the hydrogenation of ortho-xylene showed that the addition of 1.25 mol% scandium triflate accelerated the reaction by more than 30-fold, confirming the remarkable Lewis acid-induced reaction acceleration effect.
This catalytic system is effective not only for diastereoselective hydrogenation of hydrocarbon aromatic compounds and aniline derivatives, but also for diastereoselective hydrogenation of pyridine derivatives with multiple substituents, and the products can be used as synthetic intermediates for various biologically active compounds (Figure 2). It also showed high stereoselectivity in the hydrogenation of aromatic compounds containing bulky and complex chiral auxiliaries as substituents (Figure 3). In the hydrogenation reaction of aromatic compounds containing such chiral auxiliaries, the target products were obtained in high yields with one atmosphere of hydrogen by using this catalyst system, whereas the conventional method requires high temperatures and hydrogen at pressures of 50 atm or higher.
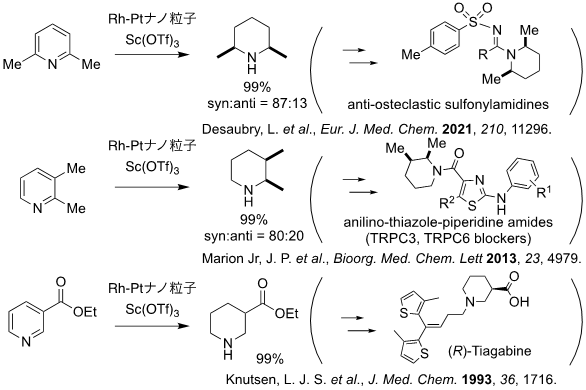
Figure 2: Synthesis of building blocks for synthesis of bulk pharmaceuticals and biologically active compounds using this catalytic system
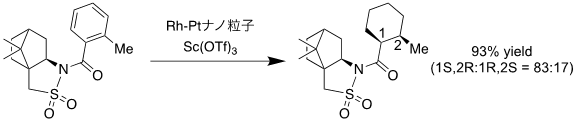
Figure 3: Stereoselective hydrogenation of aromatic compounds with chiral auxiliaries using this catalyst system
Future Development
In this study, we have developed a new catalytic system consisting of heterogeneous metal nanoparticles and Lewis acid catalysts for efficient hydrogenation of aromatic rings. This catalytic system enables smooth reactions under mild conditions even for difficult substrates that have previously required harsh reaction conditions in hydrogenation reactions. The knowledge of selective hydrogenation is also expected to open up more efficient synthetic routes for fine chemicals.
Journal
-
Journal name Angewandte Chemie , Angewandte Chemie International Edition Title of paper Reaction Rate Acceleration of Cooperative Catalytic Systems: Metal Nanoparticles and Lewis Acids in Arene Hydrogenation Author(s) Hiroyuki Miyamura *, and Shū Kobayashi * DOI Number
Terminology
Note 1 Organic hydride method
A method that uses aromatic hydrocarbons and their hydrides as a medium for the storage and transport of hydrogen. Since hydrogen is a gas, it is difficult to handle, creating storage and transportation problems. If aromatic hydrocarbons such as benzene, toluene, and naphthalene are hydrogenated and the hydrogen is immobilized in organic molecules, it can be stored and transported as a stable liquid. If a reverse reaction, dehydrogenation, is performed, hydrogen can be extracted wherever it is needed, and at the same time, the aromatic hydrocarbons can be regenerated and reused. ↑up


