DATE2022.04.11 #Press Releases
Animal morphological evolution has been limited by the "difficulty of changing body-building processes"
Disclaimer: machine translated by DeepL which may contain errors.
Yui Uchida (Doctoral student, Department of Biological Sciences, at the time of the research, now a Postdoctoral Fellow of RIKEN)
Shuji Shigenobu (Professor, National Institute for Basic Biology)
Hiroyuki Takeda (Professor, Department of Biological Sciences)
Chikara Furusawa (Professor, Universal Biology Institute / Team Leader, RIKEN)
Naoki Irie (Department of Biological Sciences / Associate Professor, Universal Biology Institute)
Key points of the presentation
- We have clarified that the potential unchangeability of the embryonic development process may have contributed to the fact that the basic anatomical features of vertebrates have remained unchanged throughout hundreds of millions of years of evolution.
- The study indicates that how animal characteristics diversify can be influenced by characteristics internalized in each organism, such as robustness to genetic and environmental changes and stability of the embryogenesis process.
- This finding provides experimental support for a theory that modifies and extends the modern theory of evolution, which explains evolution by the selection of traits that have been changed by mutation.
Summary of Presentation
Vertebrates, including humans, have diversified into various forms throughout more than 550 million years of evolution, but somehow all vertebrates have retained their basic anatomical features, the body plan (Note 1). As a background for this, it was known that the body plan formation stage of the embryogenesis process, in which a body is formed from a fertilized egg, has always been conservative throughout evolution, but the reason why it was conservative remained unclear.
A research group led by Graduate Student Yui Uchida and Associate Professor Naoki Irie at the Graduate School of Science, The University of Tokyo, based on previous theoretical work by Professor Riki Furusawa and his colleagues, hypothesized that the body plan formation stage is evolutionarily conserved because it is less prone to variation due to environmental changes, mutations, and developmental noise (Note 2), etc. Based on their previous theoretical research, Professor Sawa and his colleagues hypothesized that the body plan formation period is evolutionarily conserved because it is less prone to change (variation) due to environmental variation, mutation, and developmental noise (Note 2).
Traditionally, the evolution of organisms has been explained mainly by variations produced by mutation and subjected to natural selection, but this study showed that the low potential to produce variation may be linked to robust conservation through evolution. This is a discovery that compels an extension of modern evolutionary theory and is expected to contribute to a deeper understanding of biological evolution.
Contents of Presentation
Background of Research
Vertebrates, a group of animals that includes fish, birds, and humans, evolved over 550 million years from a common ancestor, adapting and dispersing to an extremely wide range of environments, including the sea, land, and air, and evolving a variety of forms. Despite this diversity, however, basic anatomical features (body plans) such as dorsal neural tube, ventral digestive tract, pharynx, and neural crest cell-derived organs have been maintained in all vertebrate species. Studies have shown that body plans have been maintained because the embryonic stage of body plan formation has been conserved throughout evolution (developmental hourglass model (Note 3)). However, it has not been fully understood why this developmental stage has always been conserved throughout evolution.
One is that any change in the body plan formation stage causes embryonic development to fail (leading to death) and selection to occur, while the other is that the developmental stages before and after the body plan formation stage have changed in each species because diversification is advantageous for environmental adaptation. The other hypothesis is that "each species has varied during their development before and after the body plan formation period because diversification is advantageous for environmental adaptation, but there was no particular reason for diversification during the body plan formation period. However, in recent years, a previous study by this research group (Note 4) has shown negative results for the former hypothesis, and in addition, a study (Note 5) has reported negative results for the latter hypothesis, and a new explanation has been sought.
On the other hand, previous studies by this research group had also suggested that the body plan formation stage is a relatively robust developmental stage that is unlikely to be lethal even with the addition of mutations. Furthermore, theoretical studies on the evolution of biological traits (phenotypes (Note 6 )) had reported a rule (evolutionary oscillation-response relationship (Note 7) ) that traits prone to phenotypic variation due to internal noise not caused by mutation are also prone to diversification in phenotypic evolution with mutation. Conversely, stable traits that are less prone to variation are less likely to diversify through evolution. Based on this theory and our own past research, this research group hypothesized that the developmental system during body plan formation is robust and stable, which prevents phenotypic variation, and that phenotypic diversification is unlikely to occur during evolution (Figure 1).

Figure 1: Relationship between phenotypic diversity in evolution (left) and stability of the embryogenesis process (right). Embryogenesis proceeds from the bottom to the top of the figure. The left figure shows that while phenotypic diversity among vertebrate species is high in the early and late stages of development, it is low during the body plan formation stage, the middle stage of embryonic development, and has been conserved throughout evolution. The figure on the right indicates that the phenotypic variation is smaller during body plan formation because the developmental system is more robust to genetic and environmental changes and more stable to developmental noise, which is the hypothesis tested in this study. The symbol in the center means that there is a correlation between the two.
Presentation
Past studies have shown that the body plan formation period is highly robust to environmental variation and mutation. On the other hand, the resistance to potential phenotypic variation caused by developmental noise (stability) has not been measured, and the theoretical prediction that phenotypic stability correlates with evolutionary conservatism has not yet been verified. Therefore, this research group constructed an experimental system to quantitatively evaluate the likelihood of potential phenotypic variation caused by developmental noise in the early and late stages of development, including the body plan formation period. Because previous studies have shown that gene expression patterns during body plan formation are conserved among vertebrate species, and because changes in gene expression patterns are the basis of morphological variation, the entire gene expression pattern was considered a phenotype. To evaluate the magnitude of potential phenotypic variation due to developmental noise, it is necessary to eliminate as much as possible the effects of phenotypic differences caused by genetic factors including mutations and environmental changes. The research group has developed an experimental system to evaluate phenotypic stability against developmental noise by using inbred lines of Japanese medaka (a population with nearly identical genetic backgrounds) as model animals and comparing gene expression patterns, especially in sibling embryos with perfectly matched parents, sex, birth date, and upbringing environment. In addition, we also evaluated conservation in intraspecific evolution using two wild populations of Japanese medaka to test whether developmental stages that are stable against developmental noise tend to be conserved in sub-evolution (Figure 2a).
From this experiment, we found that the "stable" developmental stage least prone to potential phenotypic variation due to developmental noise is body plan formation, and that body plan formation is highly conserved in medaka intraspecific evolution (Fig. 2b). Further investigation revealed that not only genes in the body plan formation stage, but also genes expressed in any developmental stage are more likely to be conserved in expression levels during evolution if the potential variation in gene expression levels is small (i.e., expression levels are stable). Stable genes whose gene expression levels are less likely to change tend to have smaller changes in expression levels both among wild medaka populations and among different vertebrate species (medaka vs. zebrafish, African clawed frog, and mouse), indicating that gene expression levels tend to be conserved throughout evolution (Figure 2c).
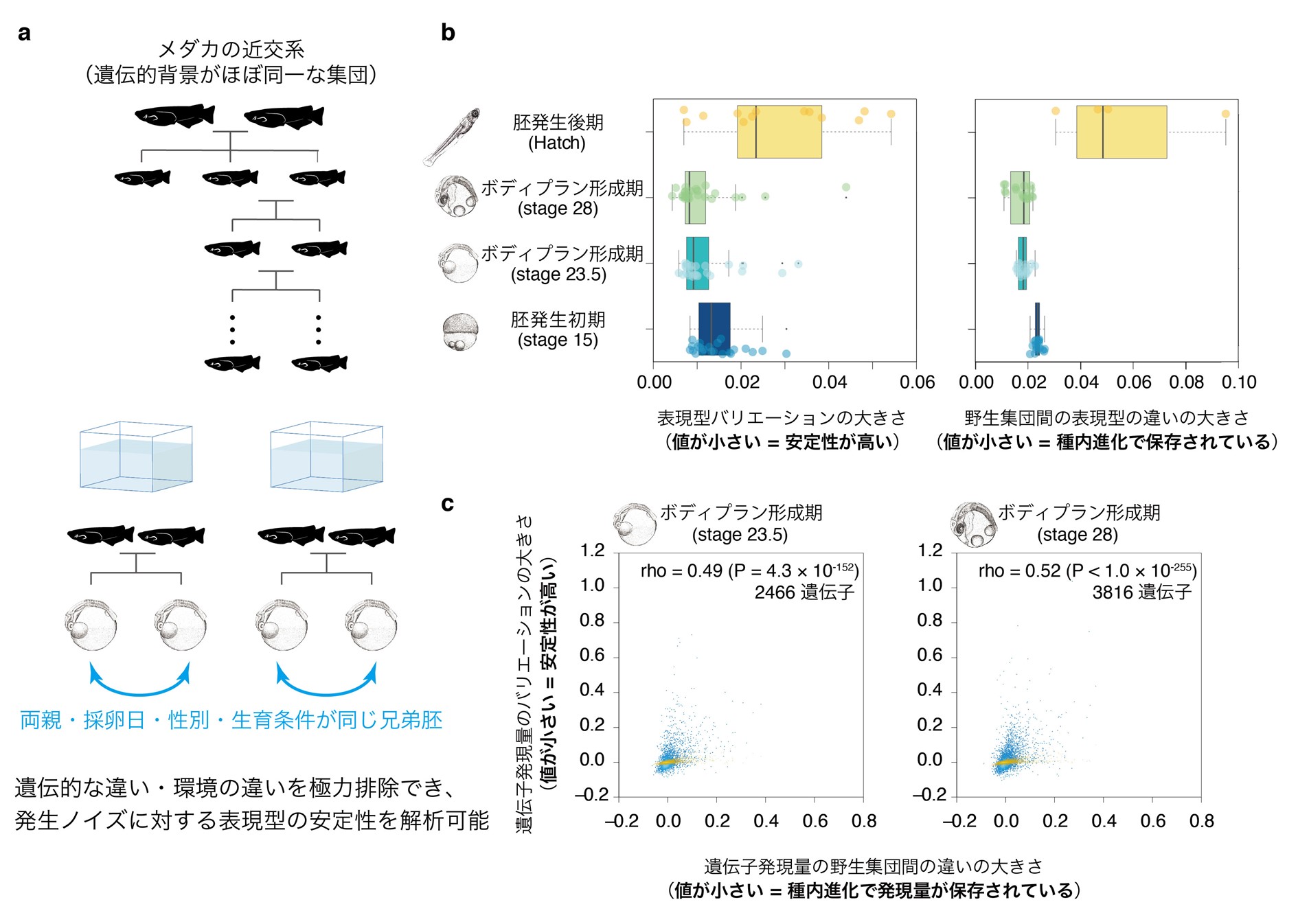
Figure 2: Experimental system constructed in this study to evaluate phenotypic stability after eliminating genetic background and environmental conditions as much as possible. Inbred lines are lines in which the genetic background between individuals is almost homogeneous through inbreeding. In order to further eliminate the effects of genetic background and environment among inbred lines, sibling embryos of the same sex born on the same day were raised in the same environment for comparative analysis. (b) Each point represents the magnitude of phenotypic variation between sibling embryos measured at each developmental stage (left) and between wild populations (right). Note that the developmental stages of high stability coincide with the most conserved stages of intraspecific evolution and body plan formation. (c) Each blue point represents the magnitude of variation in gene expression levels between sibling embryos (vertical axis) and between wild populations (horizontal axis); the correlation between the two values suggests that genes with more robust expression regulation are more likely to have their gene expression levels conserved during evolution. The yellow dots represent technical errors (vertical axis), indicating that this result cannot be explained by mere measurement technical errors.
These results are consistent with predictions from theoretical studies, indicating that a stable developmental stage in vertebrate evolution in which phenotypic variation is unlikely to occur is consistent with the body plan formation stage, which is conserved throughout evolution. This indicates that the stability of the body plan formation stage may explain the conservation of body plans (Figure 3).
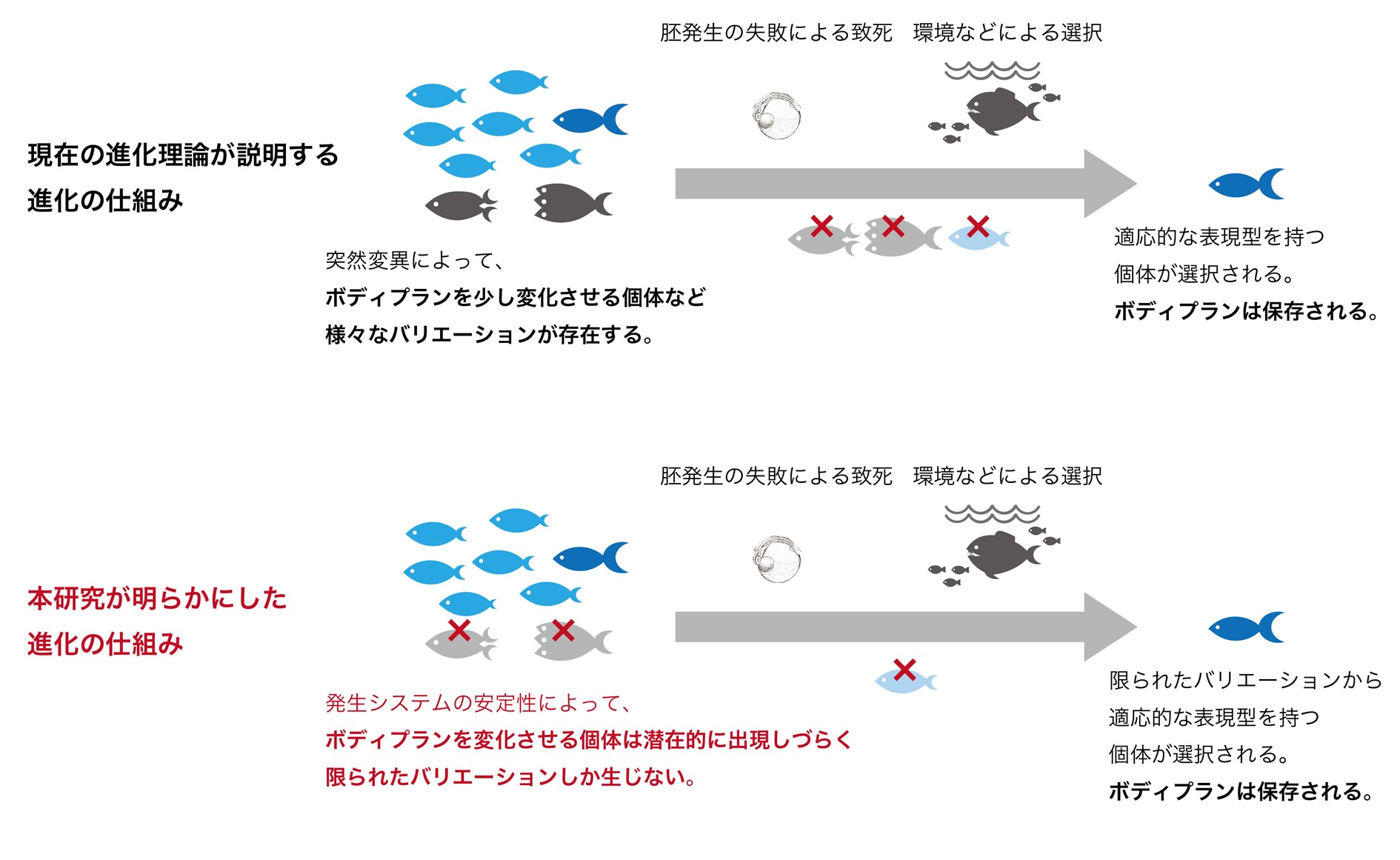
Figure 3: Extensions of conventional evolutionary theory presented in this study. Conventional evolutionary theory explains evolution by the disappearance or spread of phenotypic variation within a population due to natural selection or accidental factors. On the other hand, this study shows the possibility that before phenotypic variation occurs in a population due to mutation, there is phenotypic variation that is potentially difficult to occur due to the robustness and stability of the developmental system in the first place, and that what characteristics evolve is biased.
Significance and Prospects
Current evolutionary theory explains that the evolution of organisms occurs mainly through natural selection of phenotypic variations caused by mutations. On the other hand, this study experimentally demonstrated the possibility that the robustness and stability of the developmental process prevents phenotypic variation from occurring in the first place, which contributes to evolutionary conservatism. In other words, it is possible that intrinsic characteristics of the organism itself influence the ease or difficulty of phenotypic evolution. In the future, we hope to contribute to the extension of the current theory of evolution by elucidating the mechanisms that cause robustness and stability of developmental processes, thereby gaining a deeper understanding of the evolution of life.
Journal
-
Journal name BMC Biology Title of paper Potential contribution of intrinsic developmental stability toward body plan conservation Author(s) Yui Uchida*, Shuji Shigenobu, Hiroyuki Takeda, Chikara Furusawa, Naoki Irie*, Yui Uchida DOI Number
Terminology
Note 1 Body plan
The basic body anatomy that species belonging to a given animal phylum (phylum is one of the levels in the hierarchy of biological classification) have in common, which characterizes that animal phylum. Since vertebrates are a subphylum in the taxonomic hierarchy, their common anatomical features are referred to as bauplan to be precise (some theories refer to vertebrates as a phylum). Specifically, the spine, dorsal neural tube, ventral digestive tract, pharynx, heart, sensory organs derived from placodes, and organs derived from neural crest cells are known as components of the body plan. Some primitive vertebrate lineages have a trace spine (cirripeds) or are made of cartilage (cartilaginous fishes), and some species have a spine that is different from the so-called "backbone" made of hard bone. ↑up
Note 2: Developmental noise
Even if individuals have exactly the same genome and embryogenesis in the same environment, they do not always produce exactly the same individuals, and their phenotypes differ among individuals. This is due to stochastic and accidental behaviors in various processes, such as the molecules that make up the organism, regulation of gene expression, differentiation, and cell motility. Traits with small differences in phenotypes caused by developmental noise have stable developmental systems, while traits with large differences have unstable developmental systems. Phenotypic differences caused by developmental noise are not inherited, and it is not obvious that developmental stability affects evolution. However, theoretical studies (see Note 6) have predicted that traits with greater phenotypic variation due to noise are more likely to diversify during evolution. The present study supports the prediction of theoretical studies that highly stable phenotypes with small phenotypic variation due to developmental noise in embryogenesis are more likely to be conserved throughout evolution. ↑up
Note 3 Developmental Hourglass Model
This is one of the models that explain the general rule of relationship between embryonic development and evolution. The model states that species belonging to the same animal phylum, such as vertebrates, pass through an intermediate stage of development that maintains similarity within their taxa (conserved in evolution) and form a body plan characteristic of their taxa at this time. Recent studies based on gene expression information analysis have provided supportive results in various lineages of vertebrates, insects, and nematodes. ↑up
Note 4 Previous studies by this research group
One of the leading hypotheses to explain the conservative nature of the vertebrate body plan formation stage throughout evolution is that "even the slightest change in embryonic development during the body plan formation stage causes subsequent embryonic development to fail (leading to death) and selection," but a comprehensive study of mutations and external disturbances across a wide range of vertebrate lineages However, no study has comprehensively examined lethality due to mutation or external disturbance across a wide range of vertebrate lineages to test the hypothesis.
To test this hypothesis, previous studies by this research group have (1) identified the developmental stages during which embryonic lethality is most likely to occur due to UV irradiation-induced mutation introduction and embryo tracking, and (2) identified the developmental stages most likely to be affected by the administration of drugs that disrupt biological systems such as transcription and translation. (zebrafish, African clawed frog, and chicken, in that order). This experiment showed that embryonic mortality is most likely to occur in early development, which is different from the results obtained when the hypothesis is correct. ↑upPaper information:
Uchida, Y., Uesaka, M., Yamamoto, T., Takeda, H. & Irie, N. Embryonic lethality is not sufficient to explain hourglass-like conservation of Evodevo 9, 1-11 (2018).Note 5: Prior studies also reject the latter hypothesis.
The model that body plan formation is conservative during embryogenesis and that this conservatism contributes to the maintenance of body plans during evolution (the developmental hourglass model, see Note 3) is supported experimentally in phyla other than vertebrates, including insects, nematodes, and mollusks. One of the most popular explanations for the conservative nature of the body plan formation period is that there was no particular reason for diversification during the body plan formation period, although diversification before and after the body plan formation period has changed in each species because it is advantageous for adaptation to the environment. However, this hypothesis has not been experimentally tested. In this previous study, this hypothesis was tested in an experimental system in which a model organism, the nematode worm, was artificially evolved in a laboratory environment. The key point of the experiment was to randomly select individuals as parents of the next generation, i.e., to evolve without natural selection pressure. This study revealed that the body plan formation stage of C. elegans is preserved even when evolution is carried out in the absence of natural selection. This indicates that the effects of natural selection are not necessarily required for the body plan-forming stage. ↑up
Paper information:
Zalts, H. & Yanai, I. Developmental constraints shape the evolution of the nematode mid-developmental transition Nat. Ecol. Evol. 1, 1-7 ( 2017).Note 6 Phenotype.
A characteristic in which a gene possessed by an organism is expressed and manifested as an actual trait. Concept for genotype. ↑
Note 7 Evolutionary oscillation-response relationship
One of the laws of phenotypic evolution discovered through theoretical research. It is a relationship in which the magnitude of phenotypic change due to genetic variation or environmental perturbation (and thus the rate of evolution) and the magnitude of phenotypic change due to accidental noise are proportional. ↑up


