DATE2021.12.06 #Press Releases
Development of extracellular lactic acid biosensor
Disclaimer: machine translated by DeepL which may contain errors.
Yusuke Nasu (Assistant Professor, Department of Chemistry)
Yuki Kamijo, Department of Chemistry, Project Academic Specialist
Robert E. Campbell, Professor, Department of Chemistry
Key points of the presentation
- We have developed a biosensor (Note 3 ) eLACCO1.1 that visualizes extracellular lactate (Note 1) minimally invasively (Note 2) with green fluorescence.
- With the development of this biosensor, we have succeeded for the first time in minimally invasive visualization of the dynamics of lactic acid distributed outside of cells.
- This biosensor is expected to contribute to the elucidation of the role of lactic acid exchange between cells in the brain and other body tissues.
Summary of the Announcement
For a long time, lactate has been considered a waste product of energy production in the body through glucose metabolism. Recently, however, a theory has been proposed that lactate may be exchanged between cells and reused as an energy substance. In order to verify this theory in detail, it is necessary to minimally invasively observe the dynamics of lactate in the intercellular (i.e., extracellular) space. However, until now, there has been no method to make this possible.
Assistant Professor Yusuke Nasu, Technical Specialist Yuki Kamijo, and Professor Robert E. Campbell of the Graduate School of Science, The University of Tokyo, and their colleagues have developed a biosensor, eLACCO1 , that can visualize extracellular lactate dynamics minimally invasively with green fluorescence by using a green fluorescent protein (GFP (*4) ) and protein engineering techniques. 1, a biosensor that enables minimally invasive visualization of extracellular lactate dynamics with green fluorescence.
The theory that neurons in the brain receive lactate from neighboring cells (stellate cells) instead of glucose from the bloodstream and use lactate as their main energy source (lactate shuttle) has become one of the hot topics in brain science. eLACCO1.1 is a powerful tool for verifying the lactate shuttle and is a major enigma in brain science. eLACCO1.1 is expected to be a powerful tool to verify the lactate shuttle and to contribute to the elucidation of a major mystery in brain science.
The results of this research have been published in the British scientific journal Nature Communications.
Publication details
Background of this research
Multicellular organisms, including humans, maintain their individual activities as an aggregate of cells by supplying energy necessary for the activities of each cell (muscle cells, nerve cells, etc.) through cell-to-cell transmission of energy obtained from external sources such as food. For a long time, glucose has been considered the major intercellular energy transmitter, and lactate has been considered a mere metabolic byproduct of glucose. This negative view of lactate is still prevalent in the general public, in large part due to the classic finding in sports science of the correlation between lactate and fatigue. Recently, however, a theory has been proposed that lactate is exchanged between cells and reused as an energy substance, and the role of lactate is being reevaluated. In order to verify this new role of lactate exchanged between cells, it is necessary to observe extracellular lactate dynamics. Until now, extracellular lactate has been measured electrically using electrodes, but this method does not have sufficient spatial resolution to visualize intercellular lactate transfer, making it impossible to determine which cells are experiencing lactate concentration changes in the vicinity. Furthermore, the electrodes are inserted into the living body, which is very invasive, and the effect of biological damage on lactate dynamics has been a major concern.
Results of this research
Assistant Professor Nasu, Technical Specialist Kamijo, and Professor Campbell at the Graduate School of Science, The University of Tokyo, have developed a biosensor that changes its fluorescence intensity in a lactate-dependent manner by fusing green fluorescent protein (GFP) and lactate-binding protein TTHA0766 (Note 5) and modifying it through protein engineering in many ways. eLACCO1.1 ( extracellular lactateindicator version 1.1 ), a biosensor that changes its fluorescence intensity in a lactate-dependent manner (Figure 1). eLACCO1.1 can be introduced into biological samples as a gene, enabling lactic acid observation in biological samples with minimal invasion and high spatial resolution (Figure 2). (Fig. 2). Furthermore, together with Yurong Wen and Shuce Zhang of the University of Alberta, the X-ray crystal structure of eLACCO1 (a mutant of eLACCO1.1) was clarified (Fig. 3). The University of Tokyo group proposed a molecular mechanism for this biosensor based on this crystal structure and various mutant analyses.
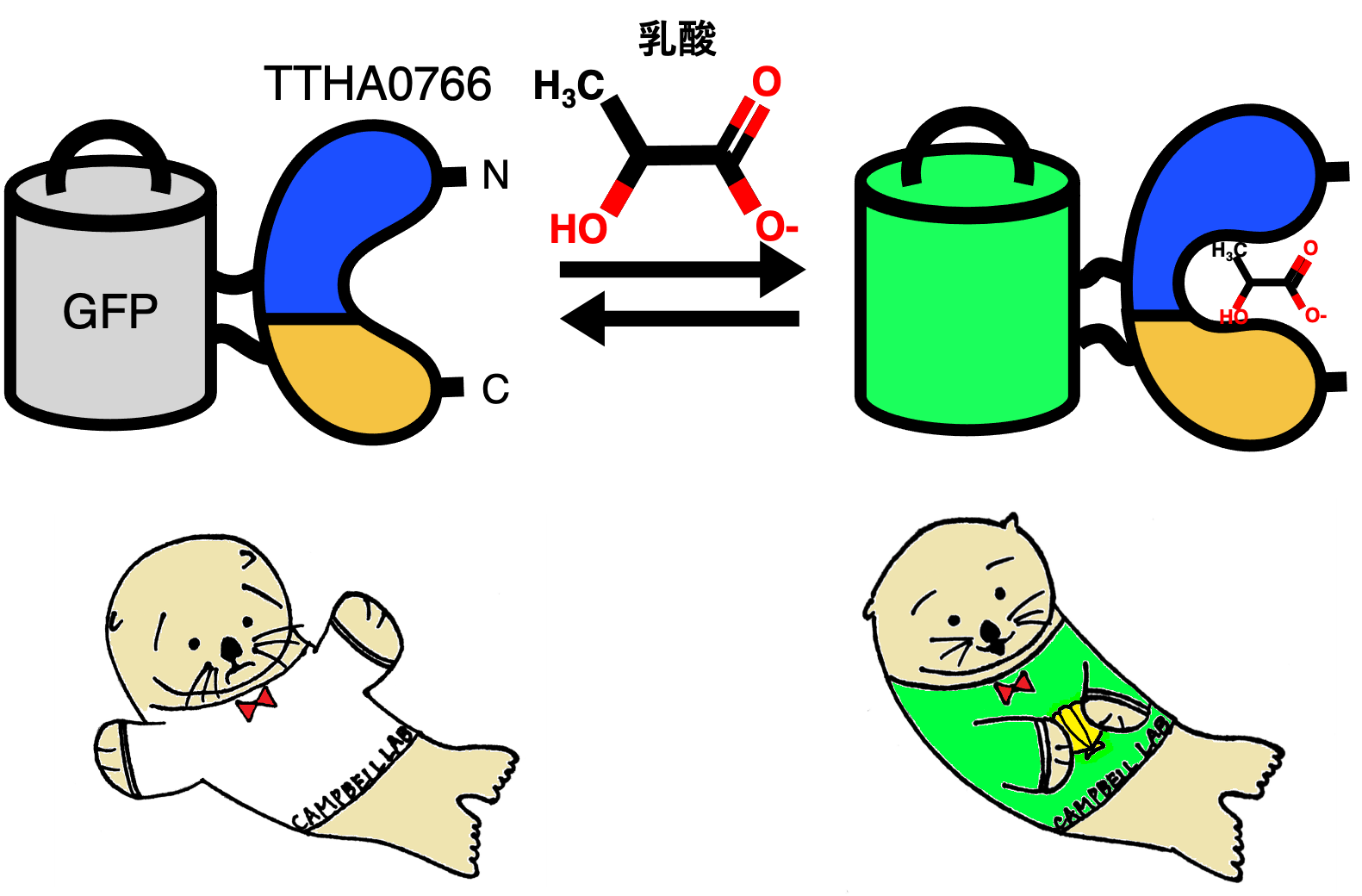
Figure 1: (Upper) Schematic of the extracellular lactate sensor eLACCO1.1, which translates conformational changes upon lactate binding to the TTHA0766 protein into changes in GFP fluorescence intensity. (Bottom) Illustration of eLACCO1.1 (designed by alumna Mina Yamane). The illustration shows sea otters capturing lactic acid (shellfish) as a source of nutrients.
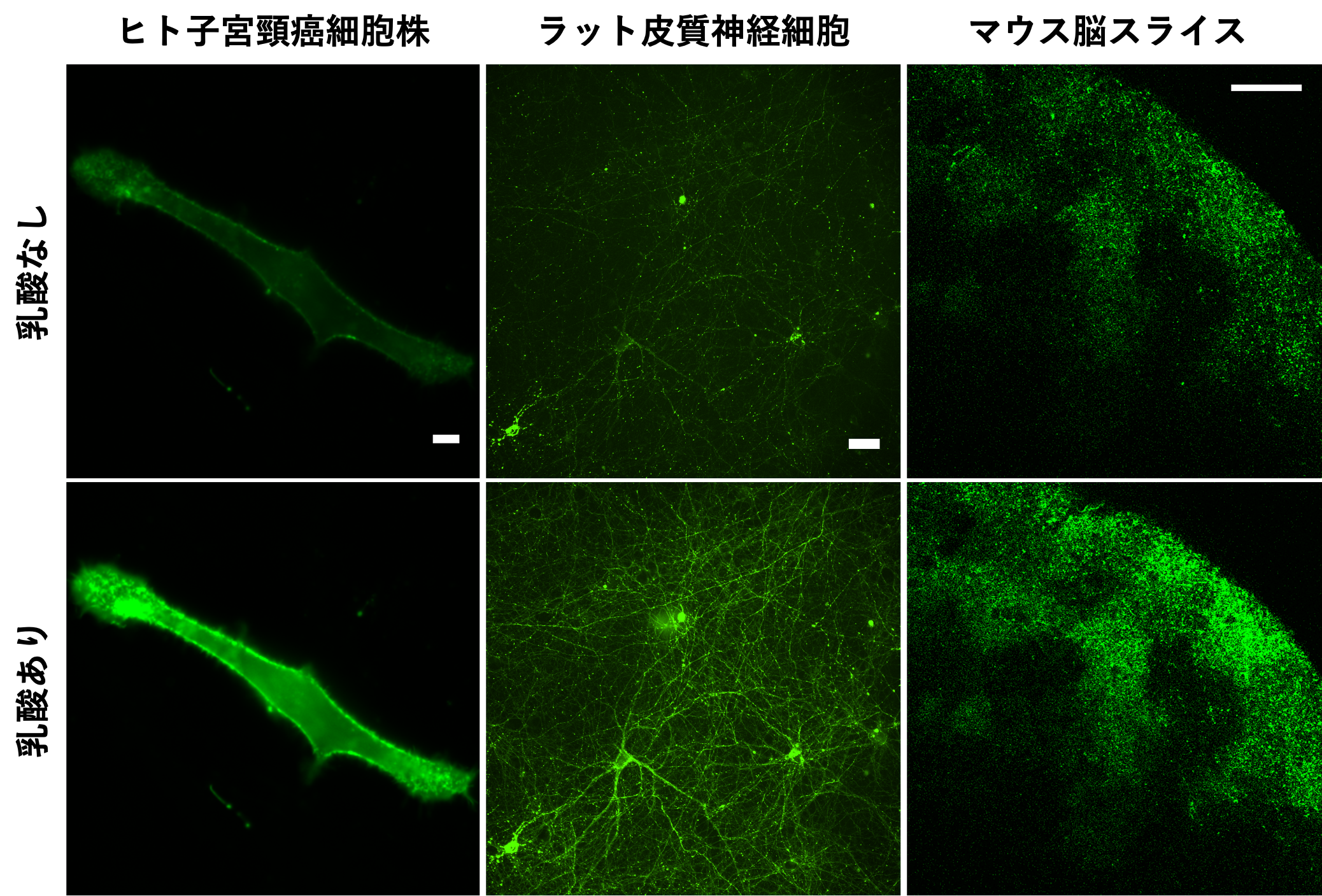
Figure 2: eLACCO1.1 fluorescence images in various biological samples. Fluorescence intensity is higher in the presence of extracellular lactate than in the absence of extracellular lactate. Extracellular lactate can be observed with cellular-level spatial resolution without invasive manipulations such as electrode insertion.
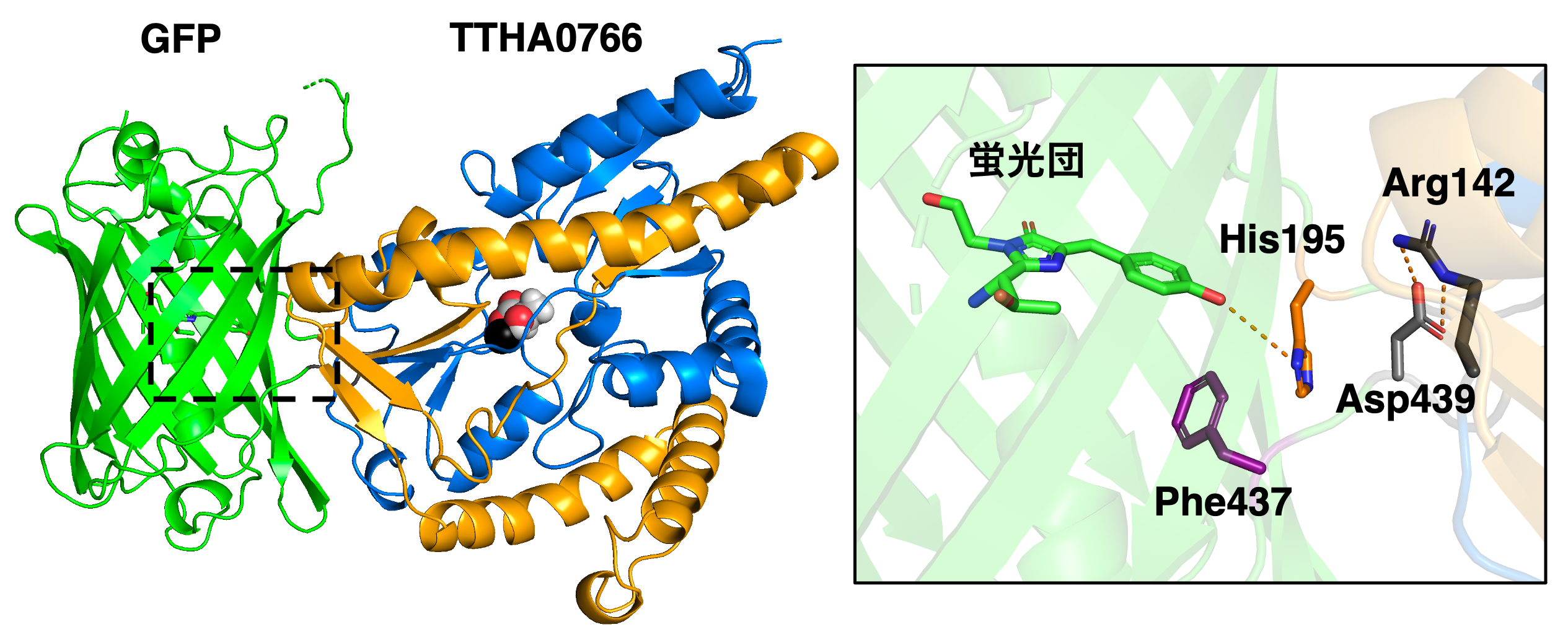
Figure 3: (Left) X-ray crystal structure of eLACCO1 in the lactate-bound state (PDB 7E9Y). Spheres indicate lactate molecules and calcium ions (black). (Right) Magnified view of the GFP fluorescent cluster. In the absence of lactate binding, the non-fluorescent state is stabilized by the interaction (hydrogen bonding) of the carboxylic side chain of the 439th aspartic acid residue (Asp439) with the fluorescent cluster. On the other hand, the conformational change of TTHA0766 associated with lactate binding causes the dissolution of the interaction between Asp439 and the fluorescent cluster by the interaction (salt bridge) between the side chain of the 142nd arginine residue (Arg142) and Asp439. Instead, the side chain of the 195th histidine residue (His195) interacts with the fluorescent cluster, stabilizing it in the fluorescent state.
Future Prospects
The theory that neurons in the brain (neurons) use lactate received from other neighboring cells (astrocytes and astrocytes) through the extracellular space as their main source of energy (lactate shuttle) has become one of the hot topics in the field of brain science. This theory overturns the conventional wisdom that neurons use glucose from the bloodstream as their primary source of energy. eLACCO1.1, a powerful tool for validating the lactate shuttle, is expected to contribute to the clarification of a major mystery in brain science and to be the beginning of a rewriting of the conventional wisdom of glucose-centered metabolism textbooks.
Acknowledgments
This study was supported by a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science, "Development of Highly Sensitive Fluorescent Lactate Sensor for Visualization of Intercellular Energy Transfer by Lactate" (project number: 19K15691, PI: Yusuke Nasu) and a Grant-in-Aid for Scientific Research (S) "Directed Evolution of a Palette The research was conducted as part of the "Directed Evolution of a Palette of Optogenetic and Chemi-Optogenetic Indicators for Multiplexed Imaging of Cellular Metabolism" (project number: 19H05633, PI: Robert E. Campbell). The research was conducted as part of the "Multiplexed Imaging for Cellular Metabolism Indicators of Cellular Metabolism" (Project Number: 19H05633 PI: Robert E. Campbell). This research was conducted in collaboration with the University of Alberta, University of Calgary, Montana State University, Howard Hughes Medical Institute, and Laval University.
Journals
-
Journal name Nature CommunicationsTitle of paper A genetically encoded fluorescent biosensor for extracellular L-lactateAuthor(s) Yusuke Nasu, Ciaran Murphy-Royal, Yurong Wen, Jordan Haidey, Rosana S. Molina, Abhi Aggarwal, Shuce Zhang, Yuki Kamijo, Marie-Eve Paquet, Kaspar Podgorski, Mikhail Drobizhev, Jaideep S. Bains, M. Joanne Lemieux, Grant R. Gordon, Robert E. Campbell*, Robert E. CampbellDOI Number 10.1038/s41467-021-27332-2Abstract URL
Terminology
Note 1 Lactate
An organic compound discovered by Swedish chemist Carl Wilhelm Scheele in 1780 in sour milk. It is generally known as a compound produced in large amounts by fatigued animal muscles and lactobacilli. ↑up
Note 2 Minimally invasive
Low damage to the target biological specimen. For example, when examining the human body, palpation is less invasive than blood sampling, which involves puncturing the skin and bleeding. up↑Note 3 Biosensor
Note 3 Biosensors
A device that measures chemical substances using the molecular identification function of living organisms. In the case of this research, it utilizes the fact that a protein derived from a bacterium called TTHA0766 recognizes lactic acid. ↑up
Note 4 Green fluorescent protein (GFP)
A protein that exhibits green fluorescence isolated in 1962 by Dr. Osamu Shimomura (2008 Nobel Prize winner in Chemistry) from the one jellyfish living in Friday Harbor, Washington, USA. The barrel-shaped protein has fluorescent clusters inside and emits green light as fluorescence when irradiated with blue light. ↑up
Note 5: Lactate-binding protein TTHA0766
In 1968, Dr. Yasuo Oshima collected a highly thermophilic bacterium Thermus thermophilis from Mine Hot Springs in Izu, Japan, and found TTHA0766, a protein capable of binding to the lactic acid present in this bacterium. ↑up


