DATE2021.03.23 #Press Releases
The process of structural change of channelrhodopsin by photo irradiation as captured by X-ray free electron laser
Disclaimer: machine translated by DeepL which may contain errors.
Kazumasa Oda (Department of Biological Sciences, 3rd year of Doctoral program)
Tomohiro Nishizawa, Associate Professor, Department of Biological Sciences
Osamu Nureki (Professor, Department of Biological Sciences)
Minoru Kubo, Professor, Graduate School of Biological Sciences, University of Hyogo
Takashi Nomura, Postdoctoral Fellow, RIKEN/ Postdoctoral Fellow, Neuroscience Research Center, RIKEN
Eriko Nango (Visiting Professor, RIKEN / Professor, Institute of Multidisciplinary Research for Advanced Materials, Tohoku University)
Sou Iwata (Group Director, RIKEN / Professor, Graduate School of Medicine, Kyoto University)
Key points of the presentation
- Using the X-ray free electron laser (Note 1), we succeeded in capturing the structural changes of channelrhodopsin, a photoreceptor protein.
- The movement of channelrhodopsin, a photoreceptor protein, induced by light irradiation was observed for the first time in the world, and the molecular basis of cation transport was successfully elucidated.
- We clarified the mechanism of ion permeation of channelrhodopsin, a photoreceptor protein, and provided fundamental information for the improvement of molecular tools using channelrhodopsin.
Summary of Presentation
Many organisms possess proteins for light acceptance. Among them, algae have a photoreceptor protein, channelrhodopsin (ChR), which causes a conformational change in the protein when it absorbs light, allowing cations to pass through ChR is used as the main tool in a technique to control neurons through light irradiation. This technology is used not only in basic research but also in various fields, including medical applications, and ChR has attracted a great deal of attention in recent years. However, the molecular basis of ChRs is still largely unknown, and in particular, the structural changes induced by light acceptance have not been reported in detail.
In this study, Professor Osamu Nureki and his group at the Graduate School of Science, The University of Tokyo, in collaboration with several laboratories in Japan and abroad, succeeded in obtaining the structural changes of ChR upon light irradiation by performing time-resolved crystal structure analysis (*2) using the X-ray free electron laser facility SACLA. This result clarified the process by which ChR becomes cation permeable upon irradiation with excitation light. The results of this study are expected to advance future research on the dynamics of photoreceptor proteins.
The results of this study were published in the online scientific journal eLife on March 23 (8 a.m. UK time).
Publication details
Many organisms possess proteins for photoreception, among which rhodopsin, a chromophore of retinal molecules, is a widely conserved membrane protein from bacteria to humans. Rhodopsin is composed of seven transmembrane helices (Note 3 ), of which retinal is attached to a lysine residue located at the seventh transmembrane helix.
Among rhodopsins, algal channelrhodopsin (ChR) isomerizes retinal upon photoacceptance, and this isomerization triggers structural changes through multiple intermediates, ultimately forming an ion-permeable pathway that transports cations. The technique of controlling neuronal activity through photo-irradiation by expressing ChRs in neurons is called optogenetics, and has become a major research method in a wide range of fields, including neurology and medicine.
While applied research using ChRs has been conducted in this way, the molecular basis of ChRs has not been well understood. Although previous studies have reported the steric structures of several ChRs and clarified basic information on the ion permeation pathways and ion selectivity of ChRs, many unknowns still remain. In particular, it is still largely unknown what kind of structural changes occur after photoirradiation to form ion transmission pathways.
Recently, with the advent of a technique called X-ray free electron laser (XFEL), a method has been developed that can reveal the structural changes of molecules in detail. In this method, crystallized proteins are stimulated by light irradiation or other stimuli, and diffraction data are collected by synchronized XFELs to capture the structural changes in the stimulated proteins. This method is called time-resolved serial femtosecond crystallography (TR-SFX: Time-Resolved Serial Femtosecond X-ray crystallography), and it is now used as a method that can capture minute structural changes of proteins with high temporal resolution (Figure 1). It is now used as a technique that can capture minute structural changes of proteins with high temporal resolution (Figure 1).
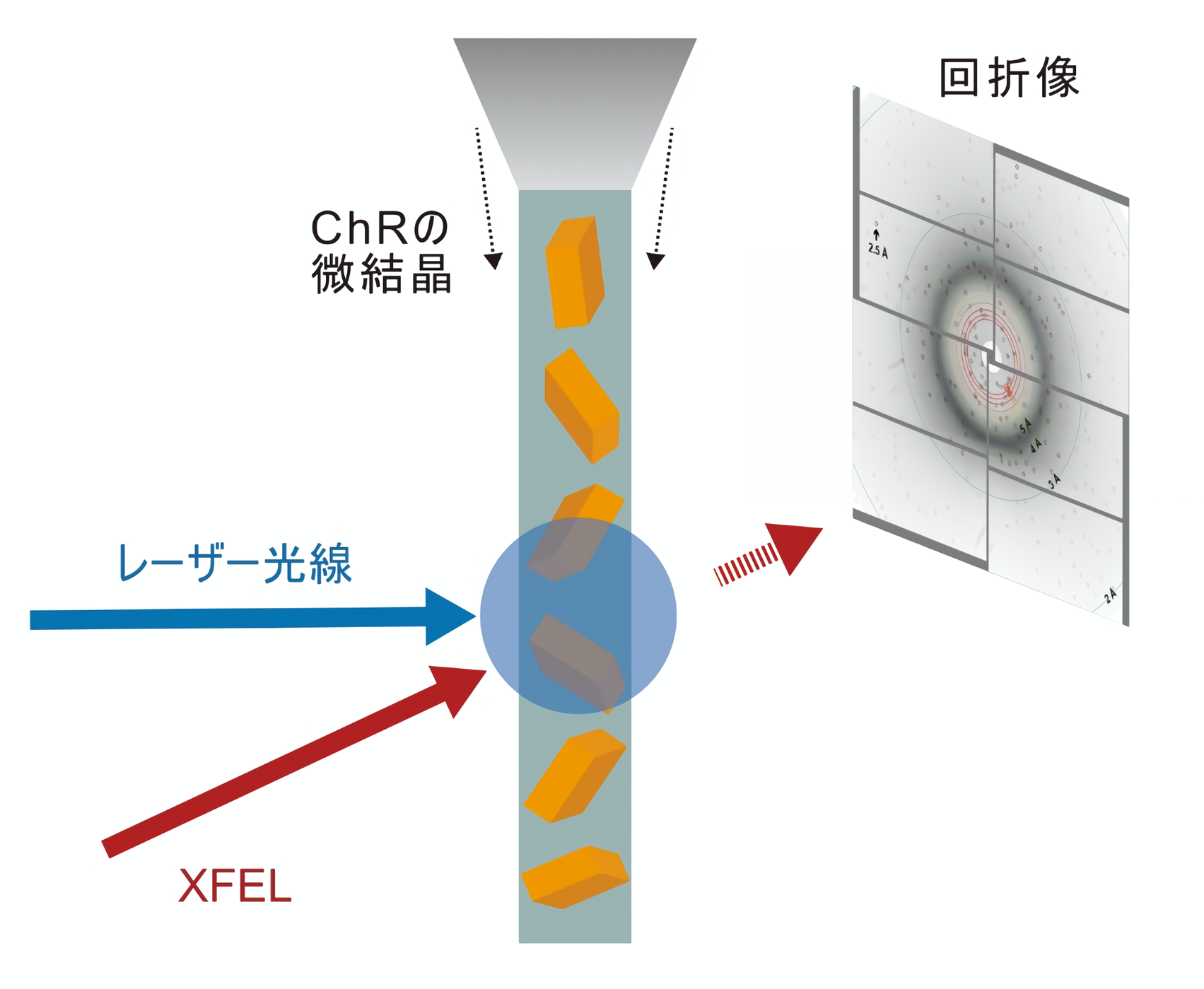
Figure 1: Schematic of time-resolved crystallography.
In this study, Professor Osamu Nureki's group at the Graduate School of Science, The University of Tokyo, in collaboration with several domestic and overseas laboratories, performed TR-SFX measurements at the X-ray free electron laser facility SACLA (Note 4) in Japan. As a result, they succeeded in capturing a part of the structural change in the process of ChRs becoming open state by photoirradiation (Movie 1).
Movie 1: Structural changes inside the ChR induced by irradiation.
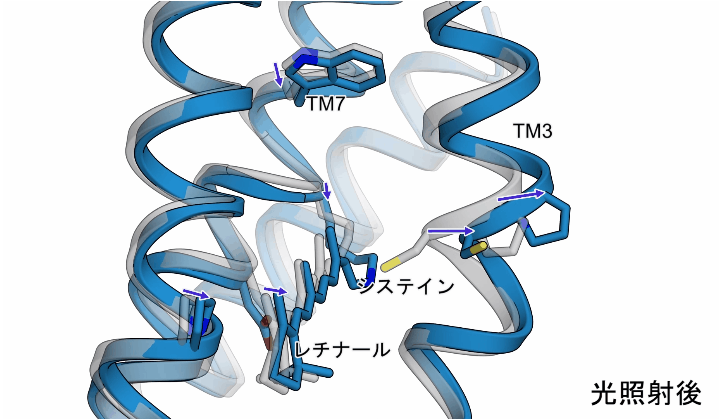
The light irradiation induced twisting movements of isomerized retinal, which pushed out the amino acid called cysteine inside the ChR, and the third transmembrane helix (TM3) containing this cysteine was observed to be pushed outward. In addition to these movements, a conformational change was also observed in the seventh transmembrane helix, to which retinal binds (Figure 2).
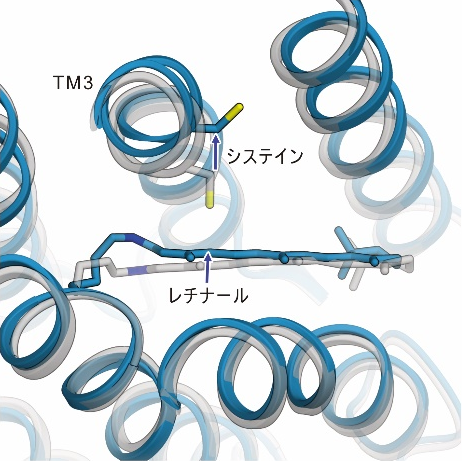
Figure 2: The structural changes in ChR observed in this study. The gray structure in the figure shows the initial state, and the blue structure shows the structure after light irradiation.
Although the structural changes observed in this study occur earlier than the actual ion influx, these transmembrane helices are thought to form ion permeation pathways, and the observed structural changes are thought to disrupt the interactions that form the gate and trigger ion influx (Fig. 3). The observed conformational change is thought to disrupt the interaction forming the gate, causing ion influx (Figure 3).
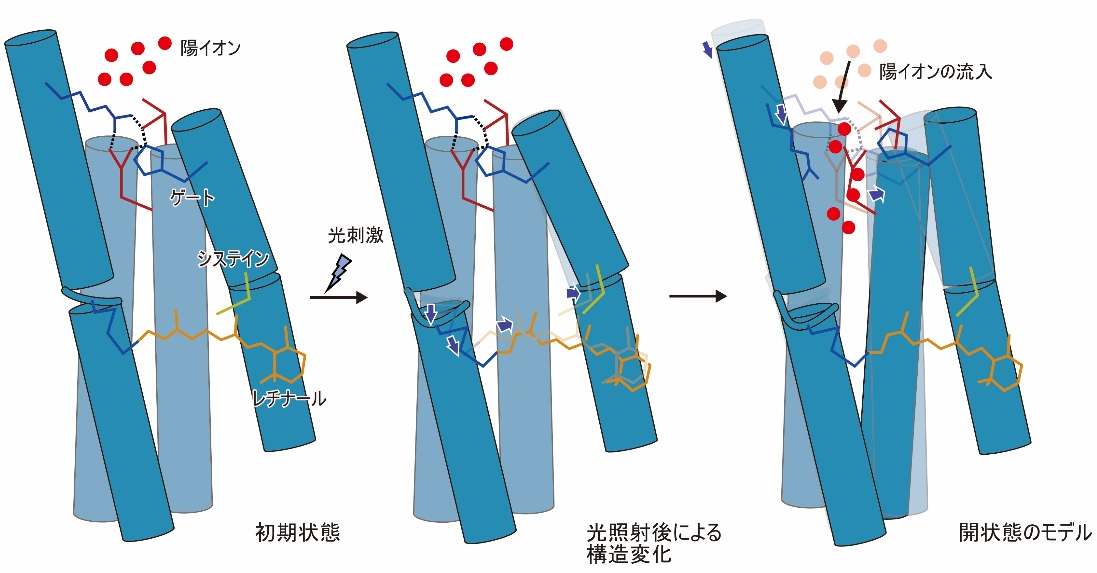
Figure 3: Model diagram of the structural changes observed in this study and the open state.
This study is one of the few to elucidate the dynamics in rhodopsin proteins, including channelrhodopsin, a photoreceptor protein, at high resolution. The elucidation of one aspect of the molecular mechanism of ion influx in channelrhodopsin is also expected to lead to the development of new tools for optogenetics.
This research was supported by the Grant-in-Aid for Specially Promoted Research "Elucidation of the Molecular Mechanism of Membrane Proteins Regulated by Physical Stimuli" (Research Project No. 16H06294, PI: Osamu Nureki) and the Grant-in-Aid for Young Scientists "Elucidation of the Open State Transition Mechanism of Channelrhodopsin Using Time-resolved Measurement" (Research Project (Research Fellow: Kazumasa Oda) and "Development of Rapid Structural Analysis Methods for Drug Target Proteins" (PI: Sou Iwata).
This research was also conducted as part of the "Drug Discovery Initiative" of the Japan Agency for Medical Research and Development (AMED), which aims to link the results of outstanding life science research to the practical application of pharmaceuticals and other products by opening up large facilities such as synchrotron radiation facilities to the outside world. The project was supported by the Basic Infrastructure Platform for Drug Discovery Support (BINDS).
Journals
-
Journal name eLife Title of paper Time-resolved serial femtosecond crystallography reveals early structural changes in channelrhodopsin Author(s) Kazumasa Oda, Takashi Nomura, Takanori Nakane, Keitaro Yamashita, Keiichi Inoue, Shota Ito, Johannes Vierock, Kunio Hirata, Andrés D. Maturana, Kota Katayama, Tatsuya Ikuta, Itsuki Ishigami, Tamaki Izume, Rie Umeda, Ryuun Eguma, Satomi Oishi, Go Kasuya, Takafumi Kato, Tsukasa Kusakizako, Wataru Shihoya, Hiroto Shimada, Tomoyuki Takatsuji, Mizuki Takemoto, Reiya Taniguchi, Atsuhiro Tomita, Ryouki Nakamura, Masahiro Fukuda, Hirotake Miyauchi, Yongchan Lee, Eriko Nango, Tomoyuki Tanaka, Rie Tanaka, Michihiro Sugawara, Tetsunari Kimura, Tatsuro Shimamura, Takaaki Fujiwara, Yasuaki Yamanaka, Shigeki Owada, Yasumasa Joti, Kensuke Tono, Ryuichiro Ishitani, Shigehiko Hayashi, Hideki Kandori, Peter Hegemann, So Iwata, Minoru Kubo*, Tomohiro Nishizawa*, Osamu Nureki*, and Shigehiko Hayashi DOI Number URL https://doi.org/10.7554/eLife.62389
Terminology
1 X-ray Free Electron Laser (XFEL)
An X-ray pulse laser with a few femtoseconds (a femtosecond is one thousand trillionth of a second) that is much more brilliant and phase aligned than the X-rays used in ordinary synchrotron radiation facilities. Since diffraction measurements can be made in a very short time, they are used for measurements such as time-resolved crystal structure analysis, in addition to being able to see structures before they are damaged by X-rays at the time of measurement. ↑up
Note 2 Time-resolved crystal structure analysis
A method of revealing changes in protein structure within a crystal by applying a specific stimulus, such as excitation light, to a protein crystal and measuring it with an X-ray free electron laser synchronized with a certain delay time. ↑up
Note 3 Transmembrane helix
In many transmembrane proteins that exist in the cell membrane, the site that penetrates the cell membrane forms a secondary structure called an alpha helix, which is called a transmembrane helix. ↑up
Note 4 X-ray free electron laser facility SACLA
The first XFEL facility in Japan, jointly constructed by RIKEN and Japan Synchrotron Radiation Research Institute (JASRI), was completed in March 2011 and named SACLA, which stands for SPring-8 Angstrom Compact free electron LAser. The first X-ray laser was emitted at SACLA in June 2011, and shared operation started in March 2012, and experiments have been conducted. Despite the compact size of the facility, which is a fraction of the size of facilities in other countries, it is capable of producing lasers with the world's shortest wavelengths of 0.1 nm or less. ↑up