DATE2022.09.27 #Press Releases
World's First Observation of Radium Dissolved in Water at the Molecular Level
Disclaimer: machine translated by DeepL which may contain errors.
- 124 years after its discovery by Curie and his wife, the dawn of radium research at the molecular level
Japan Atomic Energy Agency
Graduate School of Science, The University of Tokyo
Organization for Scientific Research on Radiation Science, Osaka University
The University of Tokyo Isotope Science Center
Summary
Dr. Eiko Yamaguchi, Member and Dr. Masahiko Okumura, Director of Simulation Technology Development Office, Center for Computational Science and Technology, Japan Atomic Energy Agency, Dr. Manya Tanaka, Director of Research Group for Environmentally Friendly Functional Materials Science, Research Center for Advanced Science and Technology, Dr. Takeshi Yaita, Vice Director of Research Center of Computational Materials Science, Osaka University, and Dr. Takashi Yoshimura, Professor of Radiation Science and Technology, Osaka University Professor Takashi Yoshimura of the Radioisotope Science Center, Osaka University, and Director Yoshio Takahashi of the Graduate School of Science/Isotope Science Center, The University of Tokyo, have succeeded in observing the structure of radium ( Ra2+ ) dissolved as an ion in aqueous solution and the surrounding water molecules (hydration structure) at the molecular level for the first time. The first successful observation of the structure of radium (Ra2+) dissolved as ions in a solution and the surrounding water molecules (hydration structure) at the molecular level. Furthermore, by using simulations, it was clarified that Ra2+ has a weaker binding force on water molecules than its homologues, and that the hydration structure is easily changed.
The radioactive element radium was discovered by Curie and his wife in 1898. Today, radium is used in drugs to treat cancer metastasized to the bones with radiation, taking advantage of the property of radium to collect in the bones formed by calcium, a homologous element to radium. Radium is also used for radiometric dating of minerals because it is produced by the decay of uranium and other elements. On the other hand, it has been pointed out that radium may contaminate the environment during drilling for underground resources such as shale gas 3) . Therefore, there is an urgent need to clarify the chemical properties of radium. However, not only is radium itself highly radioactive, but also gaseous radioactive element radon is produced by the decay of radium, increasing the risk of internal exposure. Because of these dangers, molecular-level experiments requiring high concentrations of radium could not be conducted until now, and the chemical properties of radium have remained unresolved for more than 100 years after its discovery.
In this study, we developed a measurement container that prevents radon leakage and established a method for safely preparing, transporting, and measuring high-concentration radium samples. Furthermore, by using SPring-8, one of the world's most powerful synchrotron radiation experimental facilities, we succeeded in the world's first molecular-level measurement of the Ra2+ hydration structure. In addition, by using a supercomputer to perform high-precision simulations and reproduce the experimental results, it was clarified that Ra2+ has a weaker ability to bind surrounding water molecules than its homologues, and that its hydration structure is more easily changed. These results suggest that Ra2+ is more easily taken up by living organisms and in the environment away from water than its homologues.
This study established a method for studying the chemistry of radium at the molecular level using synchrotron radiation experiments and simulations. In the future, this method will be applied to the study of more complex chemical reactions, which is expected to lead to the elucidation of the mechanism of action of cancer drugs, the development of new drugs, the refinement of dating methods for soil, the solution of environmental problems, and other important issues for society. The research results were published in the August 19 (Japan time) issue of Cell Press' iScience magazine.
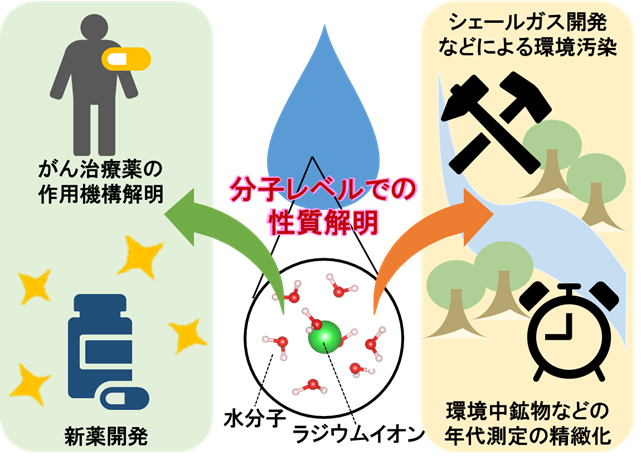
Figure: Overview of this research
For more details, please visit the website of Japan Atomic Energy Agency (JAEA).


