DATE2022.04.27 #Press Releases
Chiral catalyst and solvent repeatable asymmetric synthesis technology:
Reduced environmental impact and energy consumption with aqueous solvents
Disclaimer: machine translated by DeepL which may contain errors.
Osamu Kobayashi, Professor, Department of Chemistry
Taku Kitanosono, Assistant Professor, Department of Chemistry
Key points of the presentation
- Chiral Lewis acid catalyst (Note 2), which is widely used in catalytic asymmetric synthesis (Note 1), was recovered with solvent and successfully reused repeatedly while retaining its activity.
- The robustness of the catalyst was demonstrated in three organic reactions in water (Note 3), where catalyst deactivation due to hydrolysis is likely to occur, to show that the catalyst is at a practical level.
- This technology not only enables the reuse of the catalyst while retaining its activity, but also contributes to the reduction of energy consumption by eliminating the need for post-treatment such as distillation by recovering the catalyst with the solvent.
Summary of Presentation
Asymmetric catalysis, which enables rapid synthesis of pharmaceuticals and chemical products, is the key to green chemistry, and the ideal resource-recycling process without energy consumption and waste such as solvent distillation is required to achieve carbon neutrality. In this study, Professor Osamu Kobayashi and Assistant Professor Taku Kitanoen of the Graduate School of Science at The University of Tokyo and their colleagues have developed a waste-free asymmetric synthesis technique using water as the reaction medium, which allows the catalyst and water to be used repeatedly.
Highly active chiral Lewis acids have been deactivated during the reaction by reacting with Lewis bases such as oxygen, nitrogen, and sulfur atoms contained in organic compounds and water molecules, making it difficult to maintain their activity after the reaction. In particular, accumulation of impurities due to repeated use of the reaction solution is fatal to maintaining the activity of chiral Lewis acids, and has been the most difficult problem to solve.
In this method, a chiral scandium complex is immobilized on a polystyrene backbone, and the product, catalyst, and water can be separated only by centrifugation. In the asymmetric ring-opening reaction of mesoepoxides, the catalyst and water could be reused 10 times to efficiently obtain chiral 1,2-amino alcohols found in many pharmaceuticals. The asymmetric 1,4-addition reaction of thiols and the asymmetric Mukaiyama aldol reaction using formalin were also successfully applied.
The research results were published in the online bulletin of Angewandte Chemie and Angewandte Chemie International Edition, the journals of the German Chemical Society, at midnight on Wednesday, April 27.
Publication Details
Research Background
Against the background of concerns over the depletion of chemical resources and the environmental impact of chemical wastes, as well as efforts toward a carbon-neutral society, there is a strong need in the field of organic chemistry to reduce environmental impact by streamlining synthetic methods. Organic solvents commonly used in organic reactions are said to account for more than 80% of all chemical waste generated. Energy is also consumed in the distillation process to remove the solvent from the mixture after the reaction, and the CO2 emissions from distillation are estimated to account for 40% of the chemical industry's CO2 emissions. Although recovering and reusing both the solvent and catalyst is ideal because it simplifies the process, it was necessary to find a way to separate the solvent from the product. In particular, avoiding the accumulation of impurities in the reaction mixture was a challenge, since the catalyst is irreversibly deactivated by impurities remaining in the reaction mixture.
In particular, highly active chiral Lewis acid catalysts widely used in precise organic synthesis are easily affected by such impurities, and this has been a hindrance to the adoption of synthetic processes using them in the production of pharmaceuticals and chemical products. This is because starting materials, products, water, and other Lewis bases (Note 4) compete with each other for binding between the Lewis acid and the reacting molecule, which is essential for the reaction, and for complexation between the Lewis acid and the chiral ligand, which is necessary for maintaining the chiral environment.
To solve this problem, it is necessary to maintain a highly active asymmetric environment while inhibiting catalyst deactivation. In this study, we focused on organic synthetic aquachemistry (Note 5), which uses neither organic solvents nor surfactants, and combined it with catalyst immobilization (insolubilization) technology to achieve asymmetric synthesis where both catalyst and solvent can be used repeatedly. When water is used instead of an organic solvent, the organic compounds of the raw materials are not miscible, and the products can be easily isolated after the reaction by extraction or centrifugation operations. In addition, the highly active aqua complex (Note 6 ) can be appropriately compartmentalized (Note 7) within a hydrophobic polymer to maintain the highly active state. On the other hand, it carries the risk of catalyst functional degradation during the immobilization step or leakage of active species during the reaction. Proof-of-concept was conducted for scandium catalyst, one of the most difficult metals to both maintain high activity and selectivity and control leakage due to its high Lewis acidity.
Description of Research
In this study, scandium complexes with polystyrene-bridged chiral 2,2'-bipyridine ligands were prepared (Figure 1), and their catalytic activity was evaluated for sustainability by conducting reuse experiments in the asymmetric ring-opening reaction of mesoepoxides. The multidentate coordination framework was utilized to suppress the leakage of scandium ions, which is difficult to achieve. Centrifugation after the reaction allowed for easy separation of the product, immobilized catalyst, and water (Figure 2).
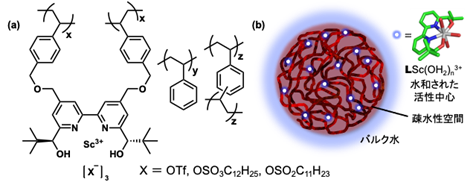
Figure 1: (a) Chiral scandium catalyst synthesized in this study, (b) image of catalyst in water
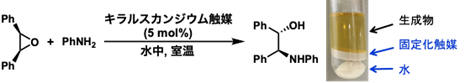
Figure 2: Asymmetric ring-opening reaction of mesoepoxides and separation of products, catalyst, and water after the reaction.
It was confirmed that the activity of the immobilized scandium complex was low when it was stirred with polystyrene, and that the catalytic activity increased significantly when immobilized in a hydrophobic environment. It was also confirmed that this immobilized catalyst is active in organic solvents and under solvent-free conditions, but the yield and selectivity are inferior to those in water. The choice of anion was effective in maintaining activity upon reuse: trifluoromethanesulfonate showed a decrease in activity after the eighth reuse, whereas dodecylsulfonate dramatically suppressed the decrease in activity and showed only a slight decrease in activity after the tenth reuse. Although the activity of undecyl sulfonate was inferior to these catalysts, no decrease in activity was observed after the 10th reuse. Since the water was used repeatedly, even a small amount of scandium ion leakage should have accumulated, but no scandium ion was detected in the water after 10 cycles of use.
It was also demonstrated that the immobilized catalyst and water could be reused in the asymmetric 1,4-addition reaction of benzyl mercaptan and the asymmetric Mukaiyama aldol reaction using formalin. In both cases, the catalyst and water were reusable in the presence of excess amounts of Lewis base relative to scandium, confirming the remarkable robustness of this catalyst system.
Future Developments
The results obtained in this study are a good example of how the characteristics of organic reactions in water can make a significant contribution to efforts in the field of organic chemistry to realize a sustainable society. In addition to the environmental friendliness, safety, and economic efficiency of water itself, the simplicity of post-treatment and the development of catalytic activity superior to that in organic solvents can bring about an innovative paradigm shift in the basic strategy for precise organic synthesis.
In the future, it is expected that the catalytic activity will be further improved and that the method will be applied to various metal catalyst species to realize a wide variety of precise organic synthesis processes.
Journal
-
Journal name Angewandte Chemie, Angewandte Chemie International Edition Title of paper Efficient Recycling of Catalyst-Solvent Couples from Lewis Acid-Catalyzed Asymmetric Reactions in Water Author(s) Taku Kitanosono,* Fangqiu Lu, Koichiro Masuda, Yasuhiro Yamashita and Shū Kobayashi* DOI Number
Glossary
1 Catalytic asymmetric synthesis
A method for synthesizing a theoretically infinite number of mirror-image isomers using a small number of chiral sources. Dr. Noyori and three other researchers were awarded the Nobel Prize in 2001. Mirror-image isomers are molecules with a molecular structure in which the mirrored image does not overlap with the original image (right-hand-left-hand relationship). Chiral (chiral) is the property of a ↑up
Note 2 Lewis acid catalyst
Lewis acid catalysts can dramatically improve reactivity by accepting electrons from oxygen, nitrogen, and sulfur atoms of reacting molecules and water molecules. By using a chiral compound as a ligand of a metal complex, the steric environment (= asymmetric environment) near the catalyst can be controlled. ↑up
Note 3: Organic reaction in water
An organic reaction that uses water as the reaction medium. Traditional organic synthesis methods often require strict anhydrous conditions, but in recent years, precise organic synthesis such as catalytic asymmetric synthesis can be achieved in water. ↑up
Note 4 Lewis base
An electron donor, as opposed to a Lewis acid. Easily reacts with Lewis acids. ↑up
Note 5 Organic synthetic aquachemistry
One method for reacting insoluble organic compounds in water is to solubilize and homogenize them by using organic solvents and large amounts of surfactants. In contrast, in methods that do not use organic solvents, raw materials react in an immiscible state with water, and may exhibit reactivity and selectivity that differ from chemical reactions that proceed under solvent-free conditions or in organic solvents. In the latest research, such cases are accumulating, and this research group refers to such organic synthesis methods that do not assume solubilization as organic synthetic aquachemistry. ↑up
Note 6 Aqua complexes
A complex with a water molecule as a ligand. Aqua complexes with a limited coordination number are known to exhibit high Lewis acidity. ↑up
Note 7 Compartmentalization
Separation from the external environment by means of a partition wall. For example, a heterogeneous catalyst with active species immobilized (insolubilized) on a solid surface is isolated from the external solvent environment. ↑up


