DATE2021.12.28 #Press Releases
If you need help, go to your siblings.
Disclaimer: machine translated by DeepL which may contain errors.
- Proteins that regulate learning behavior are supported by proteins that closely resemble -.
Shingo Hiroki (Third-year doctoral student, Department of Biological Sciences)
Yuichi Iino, Professor, Department of Biological Sciences
Key Points of the Presentation
- C. elegans (Note 1 ) is known to memorize the environment in which it was reared through the function of a protein called PKC-1. In this study, we have revealed that TPA-1, a very similar protein that is a "sibling" of PKC-1, supports the function of PKC-1 under specific conditions.
- TPA-1 has different properties than PKC-1, and learning is not normally affected by the absence of TPA-1. However, when C. elegans ages or when learning behavior is interrupted by other sensory inputs, TPA-1 is found to work to ensure that learning occurs as normally as possible.
- Proteins corresponding to PKC-1 and TPA-1 also exist in mammals, including humans, and regulate learning. The mechanism revealed in this study, in which "similar but slightly different molecules help out only when in trouble," may be a common mechanism for all organisms to learn correctly under any circumstances.
Summary of Presentation
C. elegans is known to memorize the concentration of salt around it during rearing and develop a preference for that concentration. In this behavior, the strength of the activity of a protein (enzyme) called PKC-1 has been shown to control the concentration "preference. On the other hand, it has not been fully elucidated what proteins other than PKC-1 regulate learning.
In this study, Shingo Hiroki, a graduate student, and Professor Yuichi Iino of the Department of Biological Sciences, Graduate School of Science, The University of Tokyo, found that TPA-1, a protein very similar to PKC-1, supports PKC-1 to ensure that learning occurs correctly. However, because PKC-1 and TPA-1 have slightly different shapes and characteristics, TPA-1 does not work well under normal conditions, but only under conditions that make learning difficult, such as when nerve function is impaired due to aging or when learning behavior is interrupted by extra sensory input. TPA-1 works as an aid to correct the behavior as much as possible in these conditions.
Many animals have a number of similar but slightly different sibling proteins. From the results of this study, we can infer the significance of these proteins, which may be "helping hands in times of trouble" to strengthen the mechanisms necessary for survival, such as learning. It is also known that many animals, including us, have proteins corresponding to PKC-1 and TPA-1, which also regulate learning. It is hoped that this research will lead directly to a better understanding of such systems.
Announcements
C. elegans is known to memorize the concentration of salt in its environment when it is kept in captivity, and to develop a preference for that concentration. In fact, when nematodes are placed on a salt concentration gradient, they gravitate toward the salt concentration at which they were reared. A key mechanism for this behavior is the regulation of the activity of PKC-1, a phosphatase (Note 2 ), in ASER neurons, which are salt-sensing nerves. The amount of diacylglycerol molecules decreases when the salt concentration is high and increases when the salt concentration is low, compared to the salt concentration during rearing. As the amount of diacylglycerol changes, the nematode will move toward higher concentrations when PKC-1 is more active, and will move toward lower concentrations when PKC-1 is less active. Thus, the regulation of PKC-1 activity in the salt-sensing nerve ASER causes the nematode to move toward the concentration at which it is reared.
On the other hand, proteins other than PKC-1 that function in learning have not been fully understood until now. Shingo Hiroki, a graduate student, and Professor Yuichi Iino of the Department of Biological Sciences, Graduate School of Science, The University of Tokyo, randomly damaged the gene (Note 4) in the mutant (Note 3), which has lost PKC-1 function and is always directed to lower concentrations, to obtain a mutant that is directed to higher concentrations without PKC-1. When we investigated what gene was injured to cause the mutant to move toward higher concentrations, we found that it was due to the loss of function of a gene called goa-1. Further investigation also revealed that the signal pathway (Note 5 ), the Gq pathway, which goa-1 normally suppresses, became too active, leading to higher concentrations. This was a surprise to them. This was a surprise to them, since the Gq pathway generally works to increase the amount of diacylglycerol, but until now the only known role of increased diacylglycerol in nematode learning was to enhance PKC-1 function. This led us to believe that in situations where the amount of diacylglycerol is sufficiently increased, hidden proteins other than PKC-1 may in fact be able to work in learning.
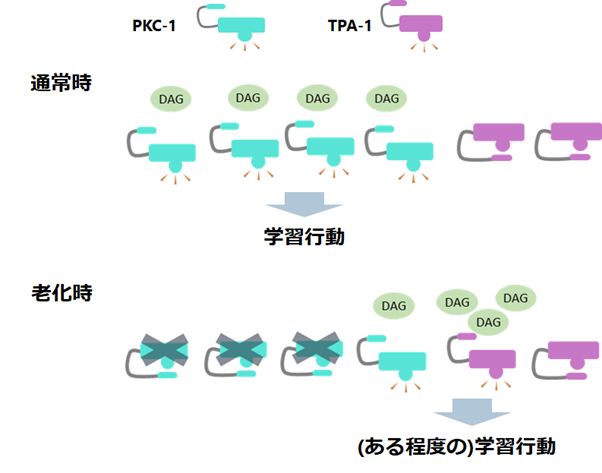
Figure 1: Under normal learning conditions, only PKC-1, which is more easily attached to diacylglycerol (DAG) fluctuations, responds preferentially, adjusting the phosphorylation of downstream proteins to appropriate levels and producing learning behavior. On the other hand, during aging, the amount of PKC-1 that can respond to diacylglycerol fluctuations is reduced. Therefore, it is thought that diacylglycerol that cannot bind to PKC-1 is attached to TPA-1, thereby generating learning behavior.
So what is the protein? Our search led us to a protein called TPA-1, which is very similar to PKC-1, a sort of "sibling" protein. The results of our experiments showed that when PKC-1 and TPA-1 were eliminated simultaneously, the nematodes would move toward lower concentrations, no matter how strongly the Gq pathway was working. Thus, it is likely that under sufficient diacylglycerol levels, TPA-1 can work in place of PKC-1 for learning. However, we also found that unlike mutants without only PKC-1, in mutants without only TPA-1, C. elegans is able to learn as usual. In other words, like their siblings, PKC-1 and TPA-1 are very similar, but there are differences. When we looked at the differences between the two proteins, we found that TPA-1 sticks to diacylglycerol less easily than PKC-1, because of differences in parts of each protein called the C1 domain and the C2-like domain (see Note 6).
Next, they looked at when TPA-1 helps learning. Here, they turned to an interesting previous study. In the hippocampus of the mammalian house mouse, (Note 7), it is known that the protein corresponding to PKC-1 becomes less active with aging, while the protein corresponding to TPA-1 does not change its activity much during aging. Inspired by this, we hypothesized that the function of PKC-1 declines during aging, but that TPA-1 provides a "helping hand" to allow normal learning to take place. When we actually investigated this, we found that when TPA-1 was eliminated in aging C. elegans, the worms showed a tendency toward lower concentrations, just as they did when PKC-1 was eliminated. This indicates that TPA-1 actually works to assist learning during aging. In addition, when we conducted other experiments under the condition that "other stimuli are introduced during the learning behavior," which makes learning difficult, we found that even under this condition, learning could not be performed normally without TPA-1.
This study revealed that TPA-1 and PKC-1, two "similar but slightly different" sibling-like molecules, assist learning only in times of trouble. Therefore, this study may lead to the elucidation of a general mechanism for stable learning. Furthermore, there are many such "similar but slightly different" proteins other than PKC. The mechanism by which these proteins "help each other out when in trouble" may be the secret for organisms to survive in various environments and under various stresses.
Journals
-
Journal name Proceedings of the National Academy of Sciences of the United States of AmericaTitle of paper The redundancy and diversity between two novel PKC isotypes that regulate learning in C. elegans.Authors Shingo Hiroki and Yuichi Iino* (co-authors)DOI number 10.1073/pnas.2106974119Abstract URL
Terminology
Note 1: Nematode
Caenorhabditis elegans, with the scientific name " Caenorhabditis elegans," is a member of a group of animals called "linear animals." In layman's terms, it is a member of the phylum Chlamydomonas and the phylum Pseudoraschidia. However, Caenorhabditis elegans is very small (about 1 mm in length) and does not parasitize animals. Instead, they live in soil and rotting fruit, feeding on bacteria and other organisms that grow there.
C. elegans is used in many studies as a model for understanding life phenomena because all neurons and their connections have been identified, its body is transparent and easy to observe, and it has an extremely rapid generation cycle of three days. ↑upNote 2: Phosphorylase called PKC-1
Phosphatases are proteins that attach what are called "phosphate groups" to specific proteins. The attachment of phosphate groups changes the function of many proteins. There are several types of phosphatases, but one of the most well-known types is PKC (protein kinase C), which can phosphorylate proteins only when it attaches to a lipid molecule, mostly diacylglycerol. In this way, PKC can translate the information that "diacylglycerol increases" into a form that "changes the function of the target protein.
PKC-1 is one type of PKC, and a more detailed classification method is called PKCε. ↑ upNote 3 Mutant
Mutants are organisms in which the DNA sequence of a gene has been altered from its normal sequence, resulting in changes such as the loss of the gene's function or, conversely, an increase in its strength. ↑up
Note 4 Gene
A gene is the physical entity, deoxyribonucleic acid (DNA), that is the blueprint for an organism to form itself. ↑up
Note 5 Signaling pathway
As mentioned in the description of 'PKC-1', PKC is able to convert changes in diacylglycerol levels into changes in the function of downstream proteins. Also, the change in diacylglycerol content is produced by the action of an enzyme called 'phospholipase C ' and phospholipase C is activated by a protein called Gq protein. Thus, the activation of one enzyme is transmitted as various changes in downstream molecules, such as Gq protein activation → phospholipase C activation → diacylglycerol increase → PKC activation → protein phosphorylation → changes in protein function. This series of pathways is called the signaling pathway. This series of pathways is called the signal pathway. ↑up
Note 6 C1, C2-like domains
The function of the C2-like domain is not clearly understood, but it is thought to function on molecules called phospholipids and to assist the function of the aforementioned C1 domain. C2-like domain is not known exactly, but it is speculated to act on molecules called phospholipids and to assist the function of the aforementioned C1 domain. ↑up
Note 7 Hippocampus
In the brains of mammals and other animals, the hippocampus is a region deep in the brain that controls memory. ↑↑


